New paper: Male sex hormones change the architecture of neurons in the brain
Sex hormones, such as estrogens and testosterone, are well known to be important for the menstrual cycle, development of sex specific characteristics, and gain of muscle mass. We understand the mechanisms behind these hormonal actions. Still subject to research is how sex hormones in the brain affect sexual behavior, aggression, and even memory. In our latest research project, carried out in Minneapolis with our collaborators, we discovered that in rats, male sex hormones change the structure of neurons in certain brain areas that are involved in the regulation of sexual behavior, motivation, and reward. These findings, published in the Journal of Neuroendocrinology , shine some light on how sex hormones might ultimately influence behavior.
Research on the effect of sex hormones in the brain is often focused on females. This is because the different phases of the menstrual cycle, which is regulated by fluctuations of sex hormone levels, dramatically affect sexual behavior in female rats. Female rats will only participate in sex during one of the menstrual cycle phases, just after levels of estrogens in the blood peaked. Previous research has shown that the high levels of estrogens cause neurons in the hypothalamus to change their structure. Because the hypothalamus is an important brain region for sexual behavior, it is therefore suggested that the structural neuronal changes partly explain why female rats become motivated for sex during the following phase of the menstrual cycle.
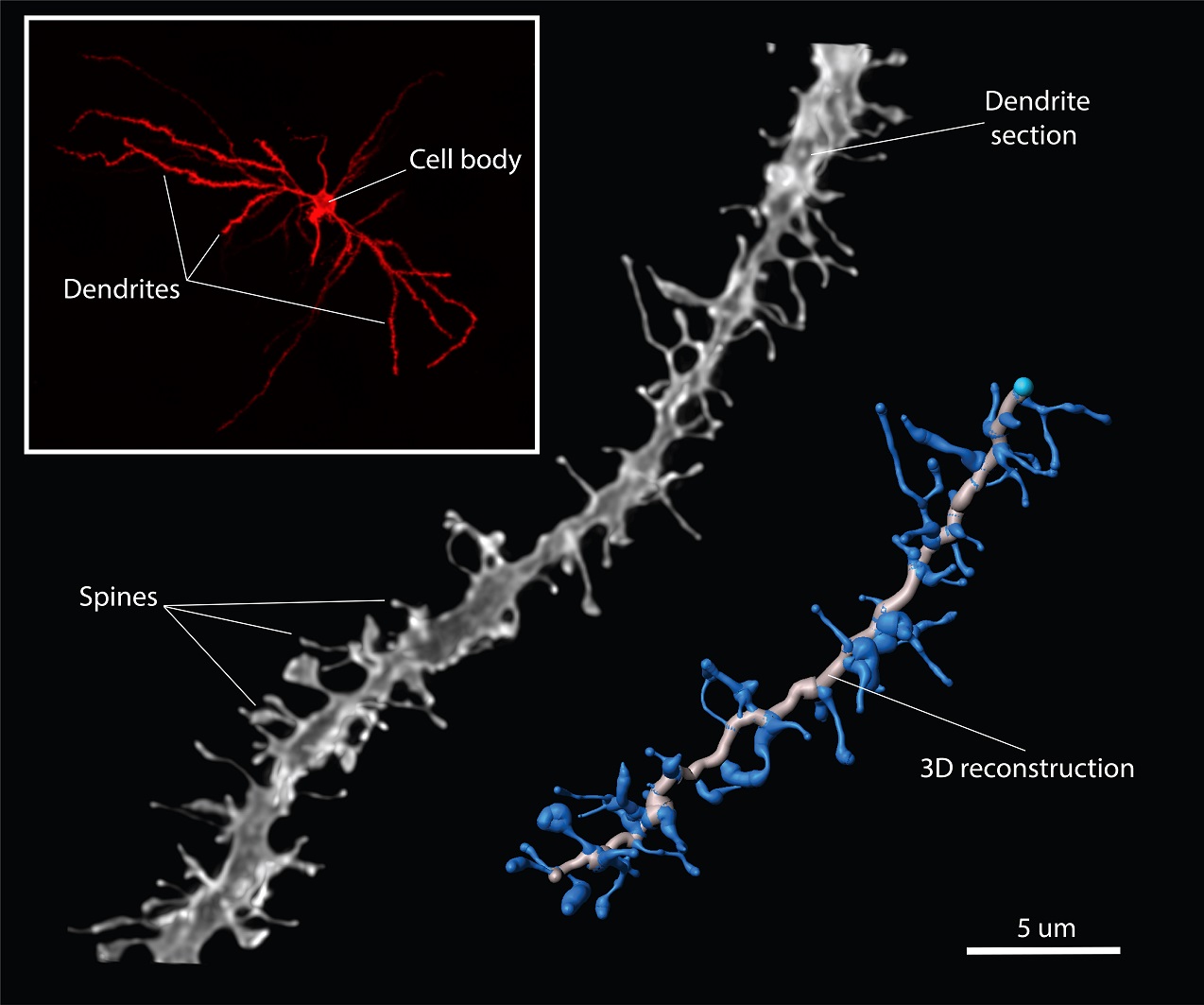
The structural neuronal changes in females during the menstrual cycle entail that the neurons become more complex due to estrogens. As shown in the picture, the structure of a neuron consists of a cell body, dendrites, and axons. The axons of a neuron connect with the dendrites of other neurons. More specifically, the contact between axons and dendrites occurs in even smaller structures on the dendrite called spines. When estrogens cause neurons in the hypothalamus of females to become more complex, it means that they increase the number of spines on the dendrites of neurons in the hypothalamus. This enables these neurons to make even more connections with other neurons. This increased connectivity in the hypothalamus caused by estrogens is necessary for female rats to be motivated for sex.
Even though males do not have a menstrual cycle, there are still fluctuations of male sex hormones during life and during certain behaviors such as aggression and sex. Therefore, the aim of our study was to find out whether male sex hormones in males can also cause the same structural changes of neurons that were found in females due to estrogens. To study this, we castrated male rats so that they would not have any sex hormones in their body and then looked at the dendrites of the neurons in different brain regions of those rats. Because neurons and their spines are tiny, we used a microscope that can take pictures of dendrites at a very high magnification and with a very high resolution. An example of such a picture is displayed here. This picture also shows a 3D reconstruction of the dendrite section that we made with the help of a specialized software program. With this 3D reconstruction, we were not only able to count the number of spines on the dendrite section, but also to determine the length and thickness of all of those spines.
We found that dendrites of neurons in the hypothalamus of the castrated male rats (which did not have any sex hormones) only had about half the number of spines compared to those of males that still had sex hormones. When we treated castrated males with dihydrotestosterone (a “male sex hormone”), the number of spines was no different from non-castrated males. This means that sex hormones are necessary for male rats to maintain the normal architecture of the neurons in the hypothalamus. We also found effects of dihydrotestosterone on neurons in the amygdala, another brain region important for sexual behavior, and in the nucleus accumbens, a brain region in the “reward system” of the brain which responds to all kinds of things that we like, such as sex. Finally, we found some evidence that shows that male sex hormones may use a similar mechanism as female sex hormones through which they affect neuronal structure.
Whether the changing number of spines caused by male sex hormones are also important for sexual motivation in males will be investigated in future research. With this study, however, we have made the first step in finding out how sex hormones might influence behavior in males. It could well be that this is regulated in a similar matter as in females.
This study was conducted in the labs of Professor Robert Meisel and Professor Paul Mermelstein at the University of Minnesota in Minneapolis, USA. The research was funded by the National Institutes of Health and the Norwegian Research Council. Patty received a personal grant from the Norwegian Research Council for her research stay in Minneapolis.
[Summary by Patty]
Huijgens PT, Snoeren EMS, Meisel RL, Mermelstein PG. Effects of gonadectomy and dihydrotestosterone on neuronal pasticity in motivation and reward related brain regions in the male rat. J Neuroendocrinol. 2020. doi: 10.1111/jne.12918
Sist oppdatert: 30.11.2020 19:57