A structural and mechanistic view on enzymes evolution
LacZymes
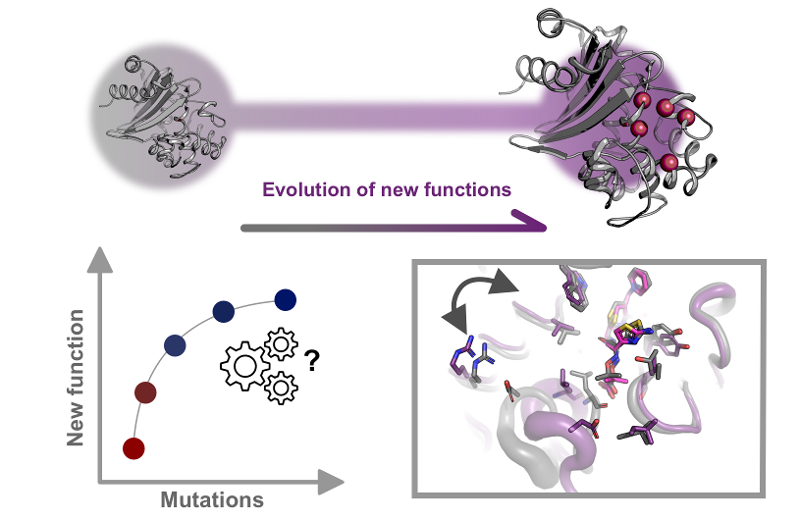

What are enzymes and their role in antimicrobial resistance development?
Enzymes are the biocatalysts of life. They are primarily proteins that accelerate chemical reactions in living organisms by lowering the activation energy required, without being consumed in the process. This catalytic efficiency enables the complex biochemical processes essential for life to occur at biologically relevant rates.
While most enzymes serve roles in metabolism, replication, and signaling, some have evolved the ability to inactivate antibiotics. These include β-lactamases, aminoglycoside-modifying enzymes, and others that chemically modify or degrade antibiotic molecules. Antibiotics like penicillin are cornerstones of modern medicine. Without them, we would lose our ability to treat life-threatening infections and carry out procedures such as cancer chemotherapy, organ transplantation, or even routine surgeries safely.
Beyond individual bacterial cells, many infections are complicated by bacterial biofilms which are structured communities of cells encased in a self-produced matrix. Biofilms are highly tolerant to antibiotics, not only due to limited drug penetration but also due to enzymes within the biofilm matrix that degrade or modify antibiotics. Some of these enzymes are constitutively expressed, while others evolve under antibiotic pressure. Enzymes also play a central role in biofilm development itself, modulating matrix composition, nutrient gradients, and signaling pathways.
Understanding the evolutionary drivers and molecular mechanisms that shape the activity of both resistance enzymes and biofilm-associated enzymes is essential. This knowledge is key to designing new antimicrobial strategies, predicting resistance trajectories, and developing drugs that can overcome both enzymatic degradation and biofilm-associated tolerance.
We are hosting Master, Bachelor and Erasmus students. Are you intersted in hands-on experience in microbiology, biochemistry and or structural biology?
Just send us an Email:
Christopher Fröhlich, christopher.frohlich@uit.no