Drug Transport and Delivery Research Group focuses on pharmaceutical technology as scientific discipline with particular interests in drug transport across biological membranes and drug delivery systems as means for improved bioavailability. The group aims at gaining a deeper understanding of transport processes of drugs in the body and its inter-relationship with drug delivery systems. The aim is the optimization of various drug dosage forms and delivery systems (advanced formulations) destined for oral, vaginal, parenteral and topical route of drug administration. Advanced drug dosage forms and delivery systems should promote the therapeutic effects of the drug and reduce its toxic effects by increasing the amount and persistence of drug in vicinity of target cells and reducing the drug exposure to non targets cells.




So what are we dealing with in the Drug Transport and Delivery research group?
DTD aims at gaining a deeper understanding of transport processes of drugs in the body and its inter-relationship with drug delivery systems. The aim is the optimization of various drug dosage forms and delivery systems (advanced formulations) destined for oral, vaginal, parenteral and topical route of drug administration. Advanced drug dosage forms and delivery systems should promote the therapeutic effects of the drug and reduce its toxic effects by increasing the amount and persistence of drug in vicinity of target cells and reducing the drug exposure to non-target cells.
Below is an overview of the main types of formulations and site of administration we are focusing on and you can read more about the different projects currently running in our group under the project descriptions.
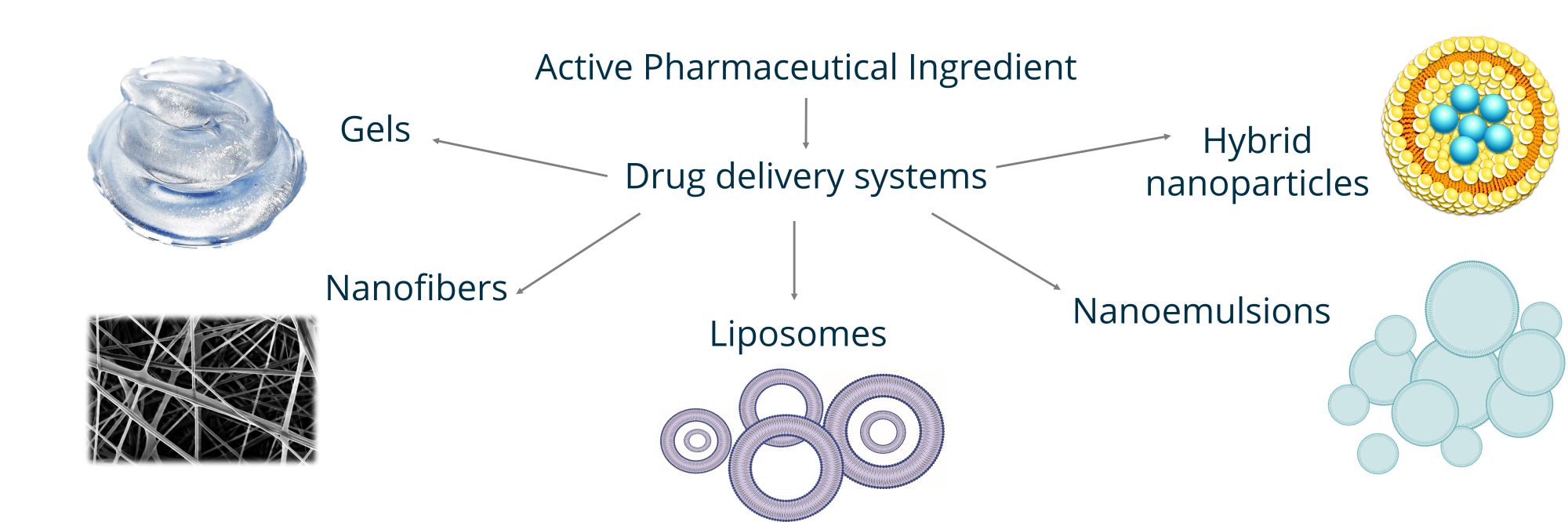 Figure showing an overview of the different types of formulations we are working with. Our main focus is the lipid based liposomal formulations, but we are also working with hydrogels, nanofibers and hybrid nanoparticles.
Figure showing an overview of the different types of formulations we are working with. Our main focus is the lipid based liposomal formulations, but we are also working with hydrogels, nanofibers and hybrid nanoparticles.
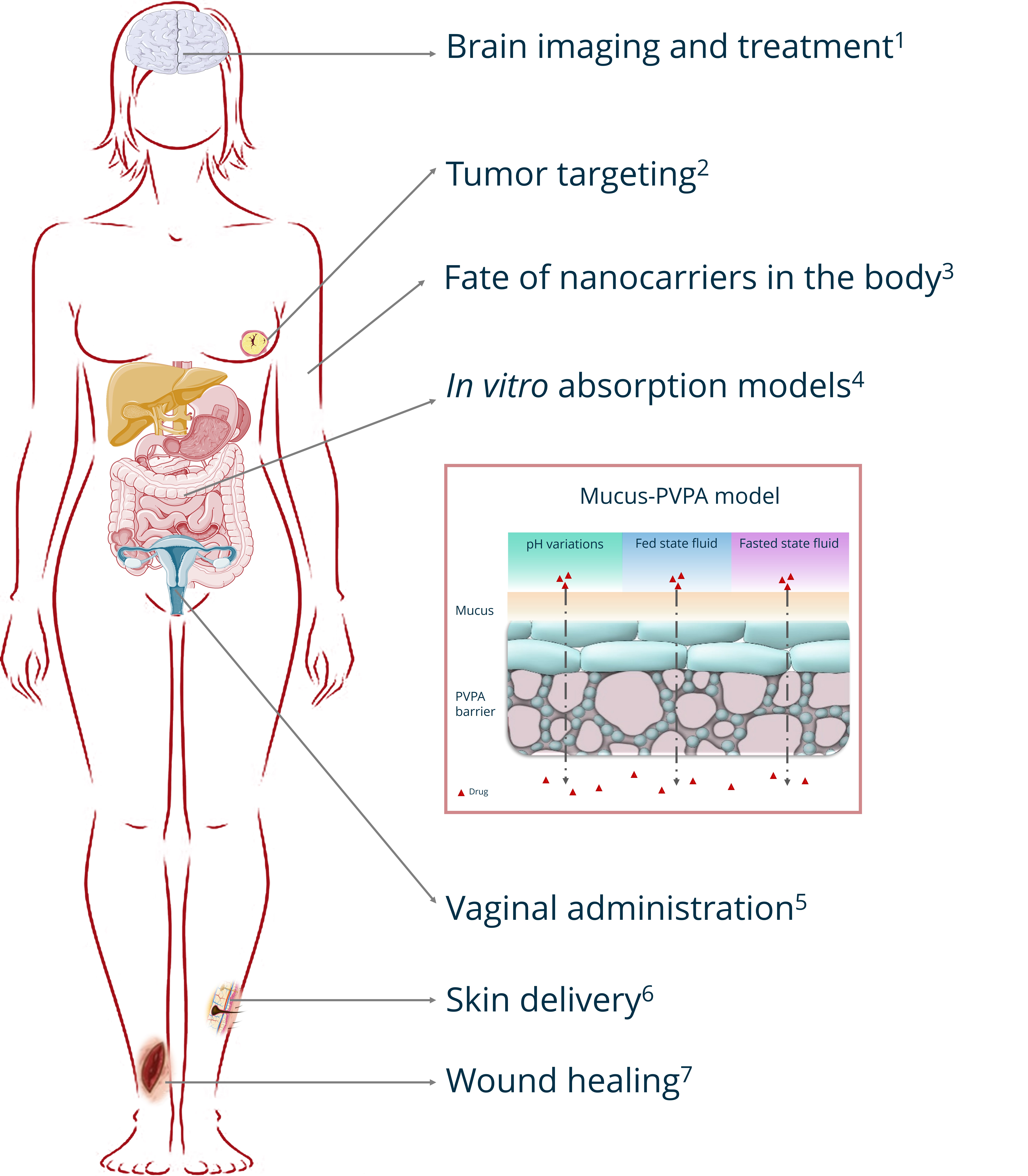 Selected publications to show the different site of administration we are working on:
Selected publications to show the different site of administration we are working on:2026
E. Markova, M. Kranz, M. Karlsen, C. Wolowczyk, A. S. Moldes Anaya, N. Škalko-Basnet, R. Sundset, A. M. Sofias, R. Berzaghi, M. Martin-Armas, S. Hak (2025)
Rapid 64Cu Radiolabeling and In Vivo Evaluation of DSPE–NODAGA Liposomes in a Murine Mammary Tumor Model, Pharmacological Research. https://doi.org/10.1016/j.phrs.2025.108065
2025
L. M. Hemmingsen, N. Škalko-Basnet (2025)
Breaking Biofilm Barriers in Skin Wounds: Membrane-Active Antimicrobials in an Era of Resistance, Current Research in Pharmacology and Drug Discovery. https://doi.org/10.1016/j.crphar.2025.100249
L. M. Hemmingsen, L. Boracchia, N. Eriksen Hagen, T. Vasskog, F. Guareschi , F. Sonvico, N. Škalko-Basnet (2025)
Lecithin-chitosan nanoparticles for co-delivery of curcumin and β-caryophyllene—potential applications in chronic wound care, Journal of Biomaterials Science, Polymer Edition, https://doi.org/10.1080/09205063.2025.2582737
A. Raza, M. Imran, N. Škalko-Basnet, S. Obuobi (2025)
Tannic acid-controlled crosslinking of albumin amyloids as multifunctional hydrogels against chronic wounds, International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2025.148707
J. Verma, F. Frejborg, M. Mantegna, V. Kumar, V. Hukkanen, G. E. Flaten, J. M. Rosenholm, K. K. Bansal (2025)
Formulation and Evaluation of Poly(jasmine lactone) based Micelles for Improving the Oral Permeability of Acyclovir, European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2025.107310
S. Mork, C. Eilertsen, N. Škalko-Basnet, and M.W. Jøraholmen (2025)
Formulation matters: Assessment of the correlation between mucoadhesiveness and type of chitosan formulation for vaginal application. Journal of Drug Delivery Science and Technology, https://doi.org/10.1016/j.jddst.2025.107564
L. V. Schulte-Werning, I. Laidmäe, L. M Hemmingsen, J Heinämäki, L. Preem, K. Kogermann, A. M. Holsæter (2025)
Antimicrobial core-shell nanofiber wound dressing with chloramphenicol-liposomes. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2025.107264
A. Raza, N. Skalko-Basnet and S. Obuobi (2025)
Alginate/Polyethylene glycol diacrylate shape memory hydrogel films for gastric retention and antibiotic delivery in H. pylori infection. Carbohydrate Polymer Technologies and Applications https://doi.org/10.1016/j.carpta.2025.100967
B. Braido, Z. Rukavina, Ø. Grimstad, S. Franzè, F. Cilurzo, Ž. Vanić, N. Škalko-Basnet and L. M. Hemmingsen (2025)
Liposomes-in-Hydrogel for Topical Drug Delivery: Mechanical, Kinetic, and Biological Insights. Journal of Drug Delivery Science and Technology. https://doi.org/10.1016/j.jddst.2025.107380
T. M. Athavale, M. Nalliah, S. Obuobi, Ø. Grimstad and N. Skalko-Basnet (2025)
Sustained release of betamethasone from solid lipid nanoparticles loaded polymeric hydrogels with natural emollient: One step closer to effective topical therapy for atopic dermatitis, Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.xphs.2025.103876
A. Sousa, R. Kulkarni, M. Johannessen, T. Wohland, N. Skalko-Basnet and S. Obuobi (2025)
Decoding interactions between biofilms and DNA nanoparticles, Biofilms, https://doi.org/10.1016/j.bioflm.2025.100260
Z. Vanic, M.W. Jøraholmen and N. Skalko-Basnet (2025)
Challenges and considerations in liposomal hydrogels for the treatment of infection. Expert Opinion on Drug Delivery, (invited review) https://doi.org/10.1080/17425247.2025.2451620
E. Markova, C. Wolowczyk, A. Mohamed, A.M. Sofias, M. Martin-Armas, R. Sundset, J. Berndtsson, S. Hak and N. Skalko-Basnet (2025).
Liposomal Nω-hydroxy-l-norarginine, a proof-of-concept: Arginase inhibitors can be incorporated in liposomes while retaining their therapeutic activity ex vivo. European Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.ejps.2024.106959
2024
S. Obuobi and N. Skalko-Basnet (2024),
Understanding vaginal biofilms: The first step in harnessing antimicrobial nanomedicine. Journal of Controlled Release, Special issue Harnessing Innovative Drug Delivery Technologies for Women's Health, Labouta H. and Benhabbour S.R. ( Eds) https://doi.org/10.1016/j.jconrel.2024.10.064
J. Rosenholm, G.E. Flaten and A. Teleki (2024),
Virtual special issue of Nordic POP: Patient-oriented products, editorial, European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2024.106737
S.Mørk, M. Johannessen, N. Škalko-Basnet and M.W. Jøraholmen (2024)
Chitosan and liposomal delivery systems for epicatechin or propyl gallate targeting localized treatment of vulvovaginal candidiasis. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2024.124489
L.V. Schulte-Werning, B. Singh, M. Johannessen, R.E. Engstad and A.M.Holsæter (2024)
Antimicrobial liposomes-in-nanofiber wound dressings prepared by a green and sustainable wire-electrospinning set-up. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2024.124136
M. Hemmingsen and N. Škalko-Basnet (2024)
Liposomes in controlled drug delivery: Controlling drug release kinetics and biodistribution/pharmacokinetics in Liposomes in Drug Delivery, What where, how and when to deliver, Ed. Sofia G. Antimisiaris, Elsevier, London, pp. 165-191. ISBN: 978-0-443-15491-1. invited chapter
I. Tho and N. Škalko-Basnet (2024)
Cell-based in vitro models for vaginal permeability studies in Concepts and Models for Drug Permeability Studies: Cell and Tissue Based In Vitro Culture Models, 2nd Ed. Editors Sarmento, Pereira, das Neves, Elsevier, Cambridge, pp. 169-186. ISBN: 978-0-443-15510-9. invited chapter
S. Das, J-C. Tinguely, S. Obuobi, N. Škalko-Basnet, K. Saxena, B. Singh Ahluwalia and D. Singh Mehta (2024)
Plasmonic nano-bowls for monitoring intra-membrane changes in liposomes, DNA nanogel and DNA micelles in suspension. Biomedical Optics Express, https://doi.org/10.1364/BOE.517471
2023
M. Bril’kov, V. Stenbakk, M. Jakubec, T. Vasskog, T. Kristoffersen, J.P. Cavanagh, J.U. Ericson, J. Isaksson and G.E. Flaten (2023)
Bacterial extracellular vesicles: towards realistic models for bacterial membranes in molecular interaction studies by Surface Plasmon Resonance. Frontiers in Molecular Biosciences, https://doi.org/10.3389/fmolb.2023.1277963
Z. Rukavina, M.W. Jøraholmen, D. Božić, I. Frankol, P.G. Gašparović; N. Škalko-Basnet, M.Š. Klarić and Ž. Vanić (2023)
Azithromycin-loaded liposomal hydrogel: a step forward for enhanced treatment of MRSA-related skin infections. Acta Pharmaceutica, https://doi.org/10.2478/acph-2023-0042
M. Giambelluca*, E. Markova*, C. Louet, B. Steinkjer, R. Sundset, N. Škalko-Basnet and S. Hak (2023)
Liposomes - human phagocytes interplay in whole blood: effect of liposome design. Nanomedicine: Nanotechnology, Biology, and Medicine, https://doi.org/10.1016/j.nano.2023.102712 (* shared first authorship)
A. Sousa, V. Borøy, A. Bæverud, K. Julin, A. Bayer, M.B. Strøm, M. Johannessen, N. Škalko-Basnet and S. Obuobi (2023)
Polymyxin B stabilized DNA micelles for sustained antibacterial and antibiofilm activity against P. aeruginosa. Journal of Materials Chemistry B, href="https://doi.org/10.1039/d3tb00704a">https://doi.org/10.1039/d3tb00704a
M. Jakubec, F.G. Rylandsholm, P. Rainsford, M. Silk, M. Bril'kov, T. Kristoffersen, E. Juskewitz, J.U. Ericson and J.S.M. Svendsen (2023)
Goldilocks dilemma: LPS works both as the initial target and a barrier for the antimicrobial action of cationic AMPs on E. coli. Biomolecules, https://doi.org/10.3390/biom13071155
L.M. Hemmingsen, V. Panzacchi, L.M. Kangu, B. Giordani, B. Luppi and N. Škalko-Basnet (2023),
Lecithin and chitosan as building blocks in anti-Candida clotrimazole nanoparticles. Pharmaceuticals, Special Issue Pharmaceutical Excipients in Formulation Design and Drug Delivery, https://doi.org/10.3390/ph16060790
A. Čačić, D.A. Klarić, S. Keser, M. Radiković, Z. Rukavina, M.W. Jøraholmen, I. Uzelac, M. Kralj, N. Škalko-Basnet, M.Š. Klarić and Ž. Vanić (2023)
A novel approach for the treatment of aerobic vaginitis: azithromycin liposomes-in-chitosan hydrogel. Pharmaceutics, Special Issue Advances in Vaginal Drug Delivery, https://doi.org/10.3390/pharmaceutics15051356
J.T. Lynnerup, J.B. Eriksen, A. Bauer-Brandl, A. M. Holsæter and M. Brandl (2023)
Insight into the mechanism behind oral bioavailability-enhancement by nanosuspensions through combined dissolution/permeation studies. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2023.106417
A. Sousa, A.N. Phung, N. Škalko-Basnet and S. Obuobi (2023)
Smart Delivery Systems for Microbial Biofilm Therapy: Carrier Design, Drug Release and Toxicology. Journal of Controlled Release, https://doi.org/10.1039/d3tb00704a
L.M. Hemmingsen, B. Giordani, M.H. Paulsen, Ž. Vanić, G.E. Flaten, B. Vitali, P. Basnet, A. Bayer, M.B. Strøm and N. Škalko-Basnet (2023)
Tailored anti-biofilm activity – liposomal delivery for mimic of small antimicrobial peptide. Biomaterials Advances, https://doi.org/10.1016/j.bioadv.2022.213238 (Journal is formerly known as Materials Science and Engineering: C; IF 8.4)
2022
L.M. Hemmingsen, P. Panchai, K. Julin, P. Basnet, M. Nystad, M. Johannessen and N. Škalko-Basnet (2022)
Chitosan-based delivery system enhances antimicrobial activity of chlorhexidine. Frontiers in Microbiology, section Infectious Agents and Disease, https://doi.org/10.3389/fmicb.2022.1023083
A.M. Holsæter, K. Wizgird, I. Karlsen, J.F. Hemmingsen, M. Brandl and N. Škalko-Basnet (2022)
How docetaxel entrapment, vesicle size, zeta potential and stability change with liposome composition–A formulation screening study. European Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.ejps.2022.106267
N. Jayakumar, F.T. Dullo, V. Dubey, A. Ahmad, F. Ströhl, J. Cauzzo, E.M. Guerreiro, O. Snir, N. Škalko-Basnet, K. Agarwal and B. Singh Ahluwalia (2022)
Multi-moded high-index contrast optical waveguide for super-contrast high-resolution label-free microscopy. Nanophotonics, https://doi.org/10.1515/nanoph-2022-0100
M.W. Jøraholmen, P. Damdimopoulou, G. Acharya and N. Škalko-Basnet (2022)
Toxicity Assessment of Resveratrol Liposomes-in-hydrogel Delivery System by EpiVaginalTM Tissue Model. Pharmaceutics, Special Issue Novel Vaginal Drug Delivery Systems, https://doi.org/10.3390/pharmaceutics14061295
E.A.L. Rustad, S. von Hofsten, R. Kumar, E.A. Lænsman, G. Berge and N. Škalko-Basnet (2022)
The pH-responsive liposomes –The effect of PEGylation on release kinetics and cellular uptake in glioblastoma cells. Pharmaceutics, A Commemorative Issue in Honor of Professor Gregory Gregoriadis: Liposomes for the Delivery of Drugs and Vaccines, https://doi.org/10.3390/pharmaceutics14061125
P. Rainsford, R.B. Sarre, B.O. Brandsdal, M. Falavigna, G.E. Flaten, M. Jakubec and J. Isaksson (2022)
WIND-PVPA: Water/Ion NMR Detected PVPA to assess lipid barrier integrity in vitro through quantification of passive water- and ion transport. BBA – Biomembranes, https://doi.org/10.1016/j.bbamem.2022.183911
S. Obuobi, A.N. Phung, K. Julin, M. Johannessen and N. Škalko-Basnet (2022)
Biofilm Responsive Zwitterionic Antimicrobial Nanoparticles to Treat Cutaneous Infection. Biomacromolecules, https://doi.org/10.1021/acs.biomac.1c01274
2021
L.M. Hemmingsen, N. Škalko-Basnet and M.W. Jøraholmen (2021)
The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Marine Drugs, invited review, https://doi.org/10.3390/md19120697
J. Grip, E. Steene, R.E. Engstad, J. Hart, A. Bell, I. Skjæveland, P. Basnet, N. Škalko-Basnet, and A.M. Holsæter (2021)
Development of a novel beta-glucan supplemented hydrogel spray formulation and wound healing efficacy in a db/db diabetic mouse model. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2021.10.013
V. Kalidasan, X. Yang, Z. Xiong, R.R. Li, H. Yao, H. Godaba, S. Obuobi, P.Singh, X. Guan, X. Tian, S.A. Kurt, Z. Li, D. Mukherjee, R. Rajarethinam, C.S. Chong, J.W. Wang, P.L.R Ee, W. Loke, B.C.K. Tee, J. Ouyang, C.J. Charles and J.S. Ho (2021)
Wirelessly operated bioelectronic sutures for the monitoring of deep surgical wounds. Nature Biomedical Engineering, https://doi.org/10.1038/s41551-021-00802-0
I. Laidmäe, A. Meos, I.A. Kjærvik, S.G. Ingebrigtsen, N. Škalko-Basnet, K. Kirsimäe, T. Romann, U. Joost, V. Kisand and K. Kogermann (2021)
Electrospun Amphiphilic Nanofibers as Templates for In Situ Preparation of Chloramphenicol-Loaded Liposomes. Pharmaceutics, https://doi.org/10.3390/pharmaceutics13111742
L.V. Schulte-Werning, A. Murugaiah, B. Singh, M. Johannessen, R.E. Engstad, N. Škalko-Basnet and A.M. Holsæter (2021)
Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-inflammatory Properties prepared by Needle-free Electrospinning. Pharmaceutics, https://doi.org/10.3390/pharmaceutics13091527
M. Šoltys, D. Zůza, T. Boleslavská, S. Akhlasová, M. Balouch, P. Kovačík, J. Beránek, N. Škalko-Basnet, G.E. Flaten and F. Štěpánek (2021)
Drug loading to mesoporous silica carriers by solvent evaporation: A comparative study of amorphization capacity and release kinetics. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2021.120982
G.E. Flaten, Ž. Vanic, N. Škalko-Basnet (2021)
The Role of In Vitro Skin Models in Optimization of Dermal Drug Delivery, in N.Dragićević and H. Maibach (eds), Percutaneous Absorption, Fifth edition, Taylor and Francis, 693-714
M.W. Jøraholmen, S. Ternullo, A.M. Holsæter, G.E. Flaten, and N. Škalko-Basnet (2021)
Advanced Drug Delivery Systems for Biologics, in H.A.E. Benson et al (eds) Fundamentals of Drug Delivery Fundamentals of Drug Delivery, First Edition, John Wiley & Sons, 231-259
Ž. Vanić, M.W. Jøraholmen and N. Škalko-Basnet (2021)
Nanomedicines for the topical treatment of vulvovaginal infections: addressing the challenges of antimicrobial resistance. Advanced Drug Delivery Reviews, invited review, https://doi.org/10.1016/j.addr.2021.113855
L.M. Hemmingsen, K. Julin, L. Ahsan, P. Basnet, M. Johannessen and N. Škalko-Basnet (2021)
Chitosomes-in-chitosan hydrogel for acute skin injuries: prevention and infection control. Marine Drugs, Special Issue Wound Healing Potential of Marine Natural Products, https://doi.org/10.3390/md19050269
J. Cauzzo, N. Jayakumar, B. Singh Ahluwalia, A. Ahmad and N. Škalko-Basnet (2021)
Characterization of Liposomes Using Quantitative Phase Microscopy (QPM). Pharmaceutics, Special issue Advances in Characterization Methods for Drug Delivery Systems, https://doi.org/10.3390/pharmaceutics13050590
L.M. Hemmingsen, B. Giordani, A.K. Pettersen, B. Vitali, P. Basnet and N. Škalko-Basnet (2021)
Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohydrate Polymers, https://doi.org/10.1016/j.carbpol.2021.117939
Z. Vinarov, B. Abrahamsson, P. Artursson, H. Batchelor, P. Berben, A. Bernkop-Schnürch, J. Butler, J. Ceulemans, N. Davies, D. Dupont, G.E. Flaten, N. Fotaki, B. Griffin, V. Jannin, J. Keemnik, F. Kesisolgou, M. Koziolek, M. Kuentz, A. Mackie, U. Maran, A.J.M. Martinez, M. McAllister, A. Müllertz, C. O’Driscoll, N. Parrott, J. Paszkowska, P. Pavek, C. Porter, C. Reppas, C. Stillhart, K. Sugano, E. Toader, K. Valentová, M. Vertzoni, S. De Wildt, C.G. Wilson and P. Augustijns (2021)
Current challenges and future perspectives in oral absorption research: an opinion of the UNGAP network. Advanced Drug Delivery Reviews, https://doi.org/10.1016/j.addr.2021.02.001
M. Falavigna, S. Brurok, M. Klitgaard and G.E. Flaten (2021)
Simultaneous assessment of in vitro lipolysis and permeation in the mucus-PVPA model to predict oral absorption of a poorly water soluble drug in SNEDDSs. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2021.120258
M. Falavigna, M. Klitgaard, R. Berthelsen, A. Müllertz and G.E. Flaten (2021)
Predicting oral absorption of fenofibrate in lipid-based drug delivery systems by combining in vitro lipolysis with the mucus-PVPA permeability model. Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.xphs.2020.08.026
Publications 2020
V. Selvarajan, S. Obuobi and P.L.R. Ee (2020)
Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Frontiers in Chemistry, https://doi.org/10.3389/fchem.2020.00602
M.W. Jøraholmen, M. Johannessen, K. Gravningen, M. Puolakkainen, G. Acharya, P. Basnet and N. Škalko-Basnet (2020)
Liposomes-in-hydrogel delivery system enhances the potential of resveratrol in combating vaginal chlamydia infection. Pharmaceutics, Special Issue on Local Antibacterial and Antimicrobial Drug Delivery Systems, https://doi.org/10.3390/pharmaceutics14061295
S. Obuobi, V. Mayandi, A. Nurul, B. Lee, R. Lakshminarayanan and P.L.R. Ee (2020)
Nucleic acid peptide nanogels for the treatment of bacterial keratitis. NANOSCALE, https://doi.org/10.1039/d0nr03095c
J. Cauzzo, M. Nystad, A.M. Holsæter, P. Basnet and N. Škalko-Basnet (2020)
Following the fate of dye-containing liposomes in vitro. International Journal of Molecular Sciences, https://doi.org/10.3390/ijms21144847
S. Obuobi and N. Škalko-Basnet (2020)
Nucleic Acid Hybrids as Advanced Antibacterial Nanocarriers. Pharmaceutics, special issue Hybrid Multifunctional Drug Delivery Systems, https://doi.org/10.3390/pharmaceutics12070643
M. Falavigna, M. Pattacini, R. Wibel, F. Sonvico, N. Škalko-Basnet and G.E. Flaten (2020)
The vaginal-PVPA: a vaginal mucosa-mimicking in vitro permeation tool for evaluation of mucoadhesive formulations. Pharmaceutics, https://doi.org/10.3390/pharmaceutics12060568
S. Obuobi, K. Julin, E.G. Fredheim, M. Johannessen and N. Škalko-Basnet (2020)
Liposomal delivery of antibiotic loaded nucleic acid nanogels with enhanced drug loading and synergistic anti-inflammatory activity against S. aureus intracellular infections. Journal of Controlled Release, https://doi.org/10.1016/j.jconrel.2020.06.002
J.Y. Ng, S. Obuobi, M.L. Chua, C. Zhang, S. Hong, Y. Kumar, R. Gokhale and P.L.R. Ee (2020)
Biomimicry of microbial polysaccharide hydrogels for tissue engineering and regenerative medicine – A review. Carbohydrate Polymers, https://doi.org/10.1016/j.carbpol.2020.116345
M. Falavigna, P.C. Stein, G.E. Flaten and M.P. di Cagno (2020)
Impact of Mucin on Drug Diffusion: Development of a Straightforward in Vitro Method for the determination of Drug Diffusivity in the Presence of Mucin. Pharmaceutics, Special Issue Mucoadhesive and Mucosal Drug Delivery Systems, https://doi.org/10.3390/pharmaceutics12020168
M.W. Jøraholmen, A. Bhargava, K. Julin, M. Johannessen and N. Škalko-Basnet (2020)
The Antimicrobial Properties of Chitosan Can be Tailored by Formulation. Marine Drugs, Special Issue Marine Chitin 2019, https://doi.org/10.3390/md18020096
F. Alopaeus, M. Hellfritzsch, T. Gutowski, R. Scherließ, A. Almeida, B. Sarmento, N. Škalko-Basnet and Ingunn Tho (2020)
Mucoadhesive buccal films based on a graft co-polymer – A mucin-retentive hydrogel scaffold. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2019.105142
B. Giordani, P. Basnet, E. Mishchenko, B. Luppi and N. Škalko-Basnet (2020)
Utilizing liposomal quercetin and gallic acid in localized treatment of vaginal Candida infections. Pharmaceutics, https://doi.org/10.3390/pharmaceutics12010009
S. Ternullo, L.V. Schulte-Werning, A.M. Holsæter and N. Škalko-Basnet (2020)
Curcumin-in-deformable liposomes-in-chitosan-hydrogel as novel wound dressing. Pharmaceutics, Special issue Semisolid Dosage Forms, https://doi.org/10.3390/pharmaceutics12010008
Publications 2019
S. Ternullo, E. Gagnat, K. Julin, M. Johannessen, P. Basnet, Ž. Vanić and N. Škalko-Basnet (2019)
Liposomes augment biological benefits of curcumin for multitargeted skin therapy. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2019.09.016
I.Y. Wu, S. Bala, N. Škalko-Basnet and M.P. di Cagno (2019)
Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2019.105026
Ž. Vanić, Z. Rukavina, S. Manner, A. Fallarero, L. Uzelac, M. Kralj, D.A. Klarić, A. Bogdanov, T. Raffai, D.P. Virok, J. Filipović-Grčić and N. Škalko-Basnet (2019)
Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. International Journal of Nanomedicine, https://doi.org/10.2147/IJN.S211691
J.H. Hansen and R. Fjellaksel (2019)
Catalytic Intermolecular Functionalization of Benzimidazoles, in M. Marinescu (eds) Chemistry and Applications of Benzimidazole and its Derivatives. IntechOpen, 119
M.P. di Cagno and P.C. Stein (2019)
Studying the effect of solubilizing agents on drug diffusion through the unstirred water layer (UWL) by localized spectroscopy. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2019.04.005
I.Y. Wu, T.E. Nikolaisen, N. Škalko-Basnet and M.P. di Cagno (2019)
The hypotonic environmental changes affect liposomal formulations for nose-to-brain targeted drug delivery. Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.xphs.2019.03.006
M. Falavigna, M. Klitgaard, E. Steene and G.E. Flaten (2019)
Mimicking regional and fasted/fed state conditions in the intestine with the mucus-PVPA in vitro model: the impact of pH and simulated intestinal fluids on drug permeability. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2019.02.035
M.W. Jøraholmen, P. Basnet, M.J. Tostrup, S. Moueffaq and N. Škalko-Basnet (2019)
Localized therapy of vaginal infections and inflammation: Liposomes-in-hydrogel delivery system for polyphenols. Pharmaceutics, Special Issue Feature Papers for 10th Anniversary of Pharmaceutics, https://doi.org/10.3390/pharmaceutics11020053
Publications 2018
R. Fjellaksel, A. Oteiza, M. Martin-Armas, P.J. Riss, O.K. Hjelstuen, S. Kuttner, J.H. Hansen and R. Sundset (2018)
First in vivo evaluation of a potential SPECT brain radiotracer for the gonadotropin releasing hormone receptor. BMC Research Notes, https://doi.org/10.1186/s13104-018-3924-2
S. Ternullo, P. Basnet, A.M. Holsæter, G.E. Flaten, L. de Weerd and N. Škalko-Basnet (2018)
Deformable liposomes for skin therapy with human epidermal growth factor: The effect of liposomal surface charge. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2018.10.005
J. Grip , R.E. Engstad, I. Skjæveland, N. Škalko-Basnet, J. Isaksson, P. Basnet and A.M. Holsæter (2018)
Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2018.05.031
T. Wagner, B. Joshi, J. Janice, F. Askarian, N. Škalko-Basnet, O.C. Hagestad, A. Mekhlif, S.N. Wai, K. Hegstad and M. Johannessen (2018)
Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins. Journal of Proteomics, https://doi.org/10.1016/j.jprot.2018.05.017
P. Berben, A. Bauer-Brandl, M. Brandl, B. Faller, G.E. Flaten, A. Jacobsen, J. Brouwers and P. Augustijns (2018)
Drug Permeability Profiling using Cell-Free Permeation Tools: An Overview and Their Applications. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2018.04.016
R. Fjellaksel, R. Sundset, J.H. Hansen and P.J. Riss (2018)
Copper-mediated late-stage iodination and 123I-labelling of triazole-benzimidazole bioactives. Synlett, https://doi.org/10.1055/s-0036-1591985
M.P. di Cagno, F. Clarelli, J. Våbenø, C. Lesley, S.D. Rahman, J. Cauzzo, E. Franceschinis, N. Realdon and P.C. Stein (2018)
Experimental determination of drug diffusion coefficients in unstirred aqueous environments by temporally resolved concentration measurements. Molecular Pharmaceutics, https://doi.org/10.1021/acs.molpharmaceut.7b01053
R. Fjellaksel, D. Dugalic, T.B. Demissie, P.J. Riss, O.K. Hjelstuen, R. Sundset and J.H. Hansen (2018)
An Acylation-Finkelstein Approach to Radioiodination of Bioactives: The Role of Amide Group Anchimeric Assistance. Journal of Physical Organic Chemistry, https://doi.org/10.1002/poc.3835
M. Falavigna, M. Klitgaard, C. Brase, S. Ternullo, N. Škalko-Basnet and G.E. Flaten (2018)
Mucus-PVPA (Mucus Phospholipid Vesicle-based Permeation Assay): an artificial permeability tool for drug screening and formulation development. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2017.12.038
Publications 2017
Ž. Vanić and N. Škalko-Basnet (2017)
Nanoformulations for Vaginal Therapy, in M. Rai and C.A. dos Santos (eds) Nanotechnology Applied to Pharmaceutical Technology, Springer International Publishing AG, pp. 183-221. ISBN 978-3-319-70298-8 ISBN 978-3-319-70299-5 (eBook)
R. Fjellaksel, M. Boomgaren, R. Sundset, I.H. Haraldsen, J.H. Hansen and P.J. Riss (2017)
Small molecule piperazinyl-benzimidazole antagonists of the gonadotropinreleasing hormone (GnRH) receptor. Medicinal Chemistry Communications, https://doi.org/10.1039/c7md00320j
S. Ternullo, L. de Weerd, A.M. Holsæter, G.E. Flaten and N. Škalko-Basnet (2017)
Going skin deep: a direct comparison of penetration potential of lipid-based nanovesicles on the isolated perfused human skin flap model. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2017.09.006
Ž. Vanić and N. Škalko-Basnet (2017)
Hydrogels for Vaginal Drug Delivery, in U.G. Spizzirri and G. Cirillo (eds) Functional hydrogels in drug delivery: key features and future perspectives. CRC Press/ Taylor & Francis Group, pp. 259-300. ISBN 9781498749015
J. Grip, R.E. Engstad, I. Skjæveland, N. Škalko-Basnet and A.M. Holsæter (2017)
Sprayable Carbopol hydrogel with soluble beta-1,3/1,6-glucan as an active ingredient for wound healing – development and in-vivo evaluation. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2017.06.029
A. Øverbye, A.M. Holsæter, M. Fusser, N. Škalko-Basnet, T.G. Iversen, M.L. Torgersen, T. Sønstevold, O. Engebraaten, K. Flatmark, G.M. Mælandsmo, T. Skotland and K. Sandvig (2017)
Ceramide-containing liposomes with doxorubicin: Time and cell-dependent effect of C6 and C12 ceramide. Oncotarget, https://doi.org/10.18632/oncotarget.20217
J. Kumari, G.E.Flaten, N. Škalko-Basnet and H. Tveiten (2017)
Molecular transfer to Atlantic salmon ovulated eggs using liposomes. Aquaculture, https://doi.org/10.1016/j.aquaculture.2017.06.019
I.Y. Wu, N. Škalko-Basnet and M.P. di Cagno (2017)
Influence of the environmental tonicity perturbations on the release of model compounds from large unilamellar vesicles (LUVs): a mechanistic investigation. Colloids and Surfaces B: Biointerfaces, https://doi.org/10.1016/j.colsurfb.2017.05.062
S.G. Ingebrigtsen, A. Didriksen, M. Johannessen, N. Škalko-Basnet and A.M. Holsæter (2017)
Old drug, new wrapping – A possible comeback for chloramphenicol? International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2017.05.025
M. Falavigna, N. Škalko-Basnet, C. Cavallari, A. Pini, B. Luppi and M.P. di Cagno (2017)
Novel in situ gel-forming solid dosage form (gfSDF) prepared by simple syringe-based moulding: a screening study. European Journal of Pharmaceutical Sciences, http://dx.doi.org/10.1016/j.ejps.2017.05.008
U. Massing, S.G. Ingebrigtsen, N. Škalko-Basnet and A.M. Holsæter (2017)
Dual Centrifugation - A Novel "In Vial" Liposome Processing Technique, in A. Catala (ed) Liposomes, IntechOpen, pp. 3-28, ISBN 978-953-51-5467-9
T. Andersen, E. Mishchenko, G.E. Flaten, J. Sollid, S. Mattsson, I. Tho and N. Škalko-Basnet (2017)
Chitosan-based nanomedicine to fight genital Candida infections: Chitosomes. Marine Drugs, https://doi.org/10.3390/md15030064
N. Škalko-Basnet and Ž. Vanić (2017)
Lipid-based Nanopharmaceuticals in Antimicrobial Therapy, in R. Boukherroub et al (eds) Functionalized Nanomaterials for the Management of Microbial Infection, A Strategy to Address Microbial Drug Resistance, 1st Edition, Elsevier, pp. 111-151. ISBN: 9780323416252
M.P. di Cagno (2017)
The potential of cyclodextrins as novel active pharmaceutical ingredients. Molecules, https://doi.org/10.3390/molecules22010001
M.W. Jøraholmen, P. Basnet, G. Acharya and N. Škalko-Basnet (2017)
PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2016.12.029
S.G. Ingebrigtsen, N. Škalko-Basnet, C. de A.C. Jacobsen and A.M. Holsæter (2017)
Successful co-encapsulation of benzoyl peroxide and chloramphenicol in liposomes by a novel manufacturing method - dual asymmetric centrifugation. European Journal of Pharmaceutical Sciences, http://dx.doi.org/10.1016/j.ejps.2016.11.017
S. Ternullo, L. de Weerd, G.E. Flaten, A.M. Holsæter and N. Škalko-Basnet (2017)
The isolated perfused human skin flap model: A missing link in skin penetration studies? European Journal of Pharmaceutical Sciences, http://dx.doi.org/10.1016/j.ejps.2016.10.003
Publications 2016
A. Konstantinell, J.A. Bruun, R. Olsen, A. Aspar, N. Škalko-Basnet, B. Sveinbjørnsson and U. Moens (2016)
Secretomic Analysis of Extracellular Vesicles Originating from Polyomavirus-Negative and Polyomavirus-Positive Merkel Cell Carcinoma Cell Lines. Proteomics, http://dx.doi.org/10.1002/pmic.201600223
E. Hagen, F.S. Løding, S. Mattsson and I. Tho (2016)
Use of interactive mixtures to obtain mini-tablets with high dose homogeneity for paediatric drug delivery. Journal of Drug Delivery Science and Technology, https://doi.org/10.1016/j.jddst.2016.03.006
A. Engesland, N. Škalko-Basnet and G.E. Flaten (2016)
In vitro models to estimate drug penetration through the compromised stratum corneum barrier, Drug Development and Industrial Pharmacy, https://doi.org/10.3109/03639045.2016.1171334
V. Staven, S. Wang, I. Grønlie and I. Tho (2016)
Development and Evaluation of a Test Program for Y-site Compatibility Testing of Total Parenteral Nutrition and Intravenous Drugs. Nutrition Journal, http://dx.doi.org/10.1186/s12937-016-0149-x
V. Staven, H. Iqbal, S. Wang, I. Grønlie and I. Tho (2016)
Physical compatibility of total parenteral nutrition and drugs in Y-site administration to children from neonates to adolescents. Journal of Pharmacy and Pharmacology, https://doi.org/10.1111/jphp.12647
S.G. Ingebrigtsen, N. Škalko-Basnet and A.M. Holsæter (2016)
Development and optimization of a new processing approach for manufacturing topical liposomes in-hydrogel drug formulations by dual asymmetric centrifugation. Drug Development and Industrial Pharmacy, http://dx.doi.org/10.3109/03639045.2015.1135940
I. Tamm, J. Heinämäki, I. Laidmäe, L. Rammo, U. Paaver, S.G. Ingebrigtsen, N. Škalko-Basnet, A. Halenius, J. Yliruusi, P. Pitkänen, S. Alakurtti and K. Kogermann (2016)
Development of suberin fatty acids and chloramphenicol loaded antimicrobial electrospun nanofibrous mats intended for wound therapy. Journal of Pharmaceutical Sciences, http://dx.doi.org/10.1016/j.xphs.2015.12.025
Publications 2015
I. Tho and N. Škalko-Basnet (2015)
Cell-based in vitro models for vaginal permeability studies, in B. Sarmento (ed) Concepts and Models for Drug Permeability Studies: Cell and Tissue Based In Vitro Culture Models. Elsevier
D. Stelzl, T.T. Nielsen, T. Hansen and M. di Cagno (2015)
Beta-CD-dextran polymer for efficient sequestration of cholesterol from phospholipid bilayers: mechanistic and safe-toxicity investigations. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2015.10.041
M.W. Jøraholmen, N. Škalko-Basnet, G. Acharya and P. Basnet (2015)
Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2015.09.007
H. Bibi, M. di Cagno, R. Holm and A. Bauer-Brandl (2015)
PermeapadTM for investigation of passive drug permeability: the effect of surfactants, co-solvents and simulated intestinal fluids (FaSSIF and FeSSIF). International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2015.07.028
I. Colzi, A.N. Troyan, B. Perito, E. Casalone, R. Romoli, G. Pieraccini, N. Škalko-Basnet, A. Adessi, F. Rossi, C. Gonnelli and S. Ristori (2015)
Antibiotic delivery by liposomes from prokaryotic microorganisms: similia cum similis works better. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2015.06.013
Ž. Vanić, A.M. Holsæter and N. Škalko-Basnet (2015)
(Phospho)lipid-based Nanosystems for Skin Administration. Current Pharmaceutical Design, Special Issue Nanotechnology for Drug Delivery, https://doi.org/10.2174/1381612821666150901095838
E. Naderkhani, T. Vasskog and G.E. Flaten (2015)
Biomimetic PVPA in vitro model for estimation of the intestinal drug permeability using fasted and fed state simulated intestinal fluids. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2015.03.017
M. di Cagno, H.A. Bibi and A. Bauer-Brandl (2015)
New Biomimetic Barrier PermeapadTM for Efficient Investigation of Passive Permeability of Drugs. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2015.03.019
M.W. Jøraholmen, G. Acharya and N. Škalko-Basnet (2015)
Kan nanopartikulære formuleringer bedre behandlingen av genitale infeksjoner? Norsk Farmaceutisk Tidsskrift, ISSN 0029-1935.
G.E Flaten, Z. Palac, A. Engesland, J. Filipović-Grčić, Ž. Vanić and N. Škalko-Basnet (2015)
In vitro skin models as a tool in optimization of drug formulation. European Journal of Pharmaceutical Sciences, https://doi.org/10.1016/j.ejps.2015.02.018
Z. Palac, J. Hurler, N. Škalko-Basnet, J. Filipović-Grčić and Ž. Vanić (2015)
Elastic liposomes-in-vehicle formulations destined for skin therapy: The synergy between type of liposomes and vehicle. Drug Development and Industrial Pharmacy, https://doi.org/10.3109/03639045.2014.938658
V. Staven, M. Waaseth, S. Wang, I. Grønlie and I. Tho (2015)
Utilization of the Tyndall effect for enhanced visual detection of particles in compatibility testing of intravenous fluids: Validity and reliability. PDA journal of pharmaceutical science and technology, https://doi.org/10.5731/pdajpst.2015.01020
T. Cerchiara, A. Abruzzo, M. di Cagno, F. Bigucci, A. Bauer-Brandl, C. Parolin, B. Vitali and B. Luppi (2015)
Micro and nanoparticulate chitosan-based systems for colon-targeted delivery of vancomycin: effect of drying methods. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2015.03.004
A. Engesland, N. Škalko-Basnet and G. E. Flaten (2015)
PVPA and EpiSkin® in Assessment of Drug Therapies Destined for Skin Administration. Journal of Pharmaceutical Sciences, https://doi.org/10.1002/jps.24315
T. Andersen, S. Bleher, G.E. Flaten, I. Tho, S. Mattsson and N. Škalko-Basnet (2015)
Chitosan in mucoadhesive drug delivery: Local vaginal therapy. Marine Drugs, https://doi.org/10.3390/md13010222
Publications 2014
Ž. Vanić and N. Škalko-Basnet (2014)
Mucosal nanosystems for improved topical drug delivery: Vaginal route of administration. Journal of Drug Delivery Science and Technology, invited review, https://doi.org/10.1016/S1773-2247(14)50085-8
Ž. Vanić, O. Planinšek, N. Škalko-Basnet and I. Tho (2014)
Tablets of pre-liposomes govern in situ formation of liposomes: Concept and potential of the novel drug delivery system. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2014.06.003
M. Hiorth, S. Nilsen and I. Tho (2014)
Bioadhesive Mini-Tablets for Vaginal Drug Delivery. Pharmaceutics, https://doi.org/10.3390/pharmaceutics6030494
M.W. Jøraholmen, Ž. Vanić, I. Tho and N. Škalko-Basnet (2014)
Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2014.06.016
S. Fulsundar, K. Harms, G.E. Flaten, P. Johnsen, B. Chopade and K. Nielsen (2014)
Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Applied and Environmental Microbiology, https://doi.org/10.1128/AEM.04248-13
E. Naderkhani, J. Isaksson, A. Ryzhakov and G.E. Flaten (2014)
Development of a biomimetic phospholipid vesicle-based permeation assay (PVPA) for the estimation of intestinal drug permeability. Journal of Pharmaceutical Sciences, https://doi.org/10.1002/jps.23954
N. Škalko-Basnet (2014)
Biologics: the role of delivery systems in improved therapy. Biologics: Targets and Therapy, invited review, https://doi.org/10.2147/BTT.S38387
Z. Palac, A. Engesland, G.E. Flaten, N. Škalko-Basnet, J. Filipović-Grčić and Ž. Vanić (2014)
Liposomes for (trans)dermal drug delivery: the skin-PVPA as a novel in vitro stratum corneum model in formulation development. Journal of Liposome Research, https://doi.org/10.3109/08982104.2014.899368
K. Berginc, S. Suljaković, N. Škalko-Basnet and A. Kristl (2014)
Mucoadhesive liposomes as new formulation for vaginal delivery of curcumin. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2014.02.006
E. Naderkhani, A. Erber, N. Škalko-Basnet and G.E. Flaten (2014)
Improved permeability of acyclovir: Optimization of mucoadhesive liposomes using the PVPA model. Journal of Pharmaceutical Sciences, https://doi.org/10.1002/jps.23845
Ž. Vanić, J. Hurler, K. Ferderber, P.G. Gašparović, N. Škalko-Basnet and J. Filipović-Grčić (2014)
Novel vaginal drug delivery system: Deformable propylene glycol liposomes-in-hydrogel. Journal of Liposome Research, https://doi.org/10.3109/08982104.2013.826242
Publications 2013
B. Thapa, I. Pepić, Ž. Vanić, P. Basnet and N. Škalko-Basnet (2013)
Topical delivery system for phytochemicals: Capsaicin and Capsicum tincture. Journal of Pharmaceutics and Drug Development, ISSN: 2348-9782
J. Hurler, K.K. Sørensen, A. Fallarero, P. Vuorela and N. Škalko-Basnet (2013)
Liposomes-in-hydrogel delivery system with mupirocin: in vitro anti-biofilm studies and in vivo evaluation in mice burn model. BioMed Research International, https://doi.org/10.1155/2013/498485
R.D. Whitaker, S.G. Ingebrigsten, E. Naderkhani, M.L. Skar and G.E. Flaten (2013)
Investigation of parameters influencing incorporation, retention and cellular cytotoxicity in liposomal formulations of poorly soluble camptothecin. Journal of Liposome Research, https://doi.org/10.3109/08982104.2013.805338
T. Andersen, Ž. Vanić, G.E. Flaten, S. Mattsson, I. Tho and N. Škalko-Basnet (2013)
Pectosomes and chitosomes as delivery systems for metronidazole: The one-pot preparation method. Pharmaceutics, Special Issue Liposome Technology, https://doi.org/10.3390/pharmaceutics5030445
J. Hurler, S. Žakelj, J. Mravljak, S. Pajk, A. Kristl, R. Schubert and N. Škalko-Basnet (2013)
The effect of lipid composition and liposome size on the release properties of liposomes-in-hydrogel. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2013.08.033
A.M. Holsæter, P. Basnet and N. Škalko-Basnet (2013)
Fra droger til moderne legemidler; hvor viktig er formuleringen? Norsk Farmaceutisk Tidsskrift, (7-8): 26-29
A. Engesland, M. Skar, T. Hansen, N. Škalko-Basnet and G.E. Flaten (2013)
New Applications of PVPA: Permeation Model Mimicking Skin Barrier. Journal of Pharmaceutical Sciences, https://doi.org/10.1002/jps.23509
G.E. Flaten, T.T. Chang, W. Phillips, M. Brandl, A. Bao and B. Goins (2013)
Liposomal Formulations of Poorly Soluble Camptothecin -Drug Retention and Biodistribution. Journal of Liposome Research, https://doi.org/10.3109/08982104.2012.742537
Ž. Vanić and N. Škalko-Basnet (2013)
Nanopharmaceuticals for improved topical vaginal delivery: Can they deliver? European Journal of Pharmaceutical Sciences, http://dx.doi.org/10.1016/j.ejps.2013.04.035
Ž. Vanić, M. Bego, A. Hafner and N. Škalko-Basnet (2013),
The characterization of various deformable liposomes with metronidazole. Drug Development and Industrial Pharmacy, https://doi.org/10.3109/03639045.2012.670247
M. Hiorth, L. Liereng, R. Reinhartsen and I. Tho (2013)
Formulation of bioadhesive Hexylaminolevulinate pellets intended for photodynamic therapy in the treatment of cervical cancer. International Journal of Pharmaceutics, https://doi.org/10.1016/j.ijpharm.2012.10.046
P. Basnet and N. Škalko-Basnet (2013)
Nanodelivery systems for improved topical antimicrobial therapy. Current Pharmaceutical Design, https://doi.org/10.2174/138161281941131219124856
P. Basnet, N. Škalko-Basnet, M.A. Hentemann and G. Acharya (2013)
Development of polyphenols-containing nanoparticles for the treatment of vaginal inflammation. Pregnancy Hypertension, https://doi.org/10.1016/j.preghy.2013.04.097
Farmasi i Tromsø har utdannet klinikere i 30 år (2025)
Beredskapsplaner: hvordan rolle skal farmasiutdanningen spille? (2025)
Sjekk datoen (2023)
20 millionar til mobilitet og samarbeid i farmasi (2023)
Antibiotikaresistens: Fant ny måte å bekjempe biofilm på (2023)
Vikingen Sigurd døde da tennene til en død mann skrapte borti leggen hans (2022)
Kvinnelig rekrutteringssuksess med egen professor-filosofi (2022)
Forskningspodcast om legemidler fra UiT Norges arktiske universitet (2018)
Hvordan velge de beste kandidatene for fremtidens legemidler? (2018)
42 millioner kroner for utvikling av persontilpasset medisin (2018)
Så lenge holder solkremen din (2017)
Er det sant at man må kaste solkremen etter ett år? (2016)
Skammelig nedprioritering (2016)
Er kvinnesykdommer mindre viktige? (2016)
Skammelig nedprioritering av forskning på kvinnesykdommer (2016)
Finner de beste legemidlene – uten dyreforsøk (2015)
Gjennombrudd: Dyreforsøk med brannskader kan erstattes (2014)
Hermer stoffopptak via hud og tarm (2012)
Legemidler og barrierer (2012)
This bacterium outsmarts our defences, but we are on its heals (2025)
Bacterial membrane satellites prove important testing ground for new antibiotics (2024)
Bacteria have a new mortal enemy: DNA (2024)
Bacterial membrane satellites prove important testing ground for new antibiotics (2024)
Nanocarriers are the Trojan horses of antibiotics (2022)
The day that Sigurd the Wiking died from a bacterial infection (2022)
Drugs and barriers (2012)
Pulver (2025)
Gel (2025)
Nesespray (2025)
Preformulering (2025)
Hetteglass (2025)
Legemiddelform (2025)
Galenisk farmasi (2025)
Dose (2025)
Farmakopé (2024)
Inhalator (2024)
Depopreparater (2024)
Den Europeiske Farmakopé (2024)
Norske legemiddelstandarder (2024)
Forstøverapparat (2024)
Hardfett (2023)
Kapsler (2023)
Stikkpiller (2023)
Vagitorier (2023)
Vaginalring (2023)
Pasta (2023)
Magistrell forskrivning (2023)
Pille (2023)
Dosepulver (2023)
Salve (2023)
Krem (2023)
Lotion (2023)
Talkum (2023)
Drasjere (2023)
Liniment (2022)
Tabletter (2022)
Silje Mork, 2021- 2025, UiT The Arctic University of Norway, Advanced liposomal delivery systems for polyphenols to combat vulvovaginal candidiasis: Tailoring chitosan to optimize mucoadhesion, Main supervisor Jøraholmen, Co-supervisor Skalko-Basnet
Elena Markova, 2021-2025, UiT The Arctic University of Norway, Eat, Deliver, Destroy: How Liposomes and Phagocytes Team Up, Harnessing liposome - phagocyte interactions for enhanced drug delivery, imaging, and cancer immunotherapy, Main supervisor Rune Sundset, Co-supervisor Skalko-Basnet (shared project with Department of Clinical Medicine, UiT)
Alexandra Sofia Antunes De Sousa 2021-2025, University of Tromsø The Arctic University of Norway, Nucleic acids against biofilms: DNA nanoparticles as drug delivery systems for biofilm penetration, inhibition and eradication, Main supervisor Obuobi, Co-supervisor Skalko-Basnet
Laura Victoria Schulte-Werning 2019-2025, University of Tromsø The Arctic University of Norway, Innovative nanofiber systems as antimicrobial wound dressings: The impact of biopolymers, liposomes and varying nanofiber complexities on chloramphenicol delivery, Main supervisor Holsæter, Co-supervisor Skalko-Basnet
Lisa Myrseth Hemmingsen 2018-2022, University of Tromsø The Arctic University of Norway, Advanced topical delivery systems for membrane-active antimicrobials. Exploring nature to improve antimicrobial wound therapy. Main supervisor Skalko-Basnet, Co-supervisor Eide Flaten
Jennifer Cauzzo 2018-2022, University of Tromsø The Arctic University of Norway, Microscopy Meets Nanomedicine: The Challenge of Liposomes, Selecting, Understanding, and Adapting Imaging Techniques to Localize and Characterize Nanocarriers. Main supervisor Skalko-Basnet, Co-supervisor Holsæter
Margherita Falavigna, 2017-2021, University of Tromsø The Arctic University of Norway, The development journey of an artificial intestinal model predicting oral drug absorption: the mucus-PVPA model. Main supervisor Eide Flaten, Co-supervisor Skalko-Basnet
Iren Wu Yeeling, 2015-2019, University of Tromsø The Arctic University of Norway, Development of osmotically active liposomes for nose-to-brain drug delivery. Main supervisor di Cagno, Co-supervisor Skalko-Basnet
Jostein Grip 2014-2018, University of Tromsø The Arctic University of Norway, Development of novel wound dressing with soluble beta-glucan (SBG®) as an active ingredient. Main supervisor Holsæter, Co-supervisors Skalko-Basnet and Engstad
Sveinung Ingebrigtsen 2012-2018, University of Tromsø The Arctic University of Norway, Novel dual centrifugation manufacturing method for liposomes-in-hydrogel: Potential in antimicrobial skin therapy. Main supervisor Holsæter, Co-supervisor Skalko-Basnet
Selenia Ternullo 2014-2018, University of Tromsø The Arctic University of Norway, Nanocarriers for tailored skin delivery: More than just the carriers? Main supervisor Skalko-Basnet, Co-supervisors Eide Flaten, Holsæter and de Weerd
May Wenche Jøraholmen 2012-2015, University of Tromsø, Surface modification of liposomes increases drug efficacy in local vaginal therapy. Main supervisor Skalko-Basnet, Co-supervisors Basnet, Acharya and Tho
Vigdis Staven 2012-2016, University of Tromsø, Y-site compatibility testing of intravenous drugs and total parenteral nutrition Establishment of a test program and study of mixtures relevant for children. Main supervisor Ingunn Tho, Co-supervisor Holsæter
Elenaz Naderkhani 2011-2015, University of Tromsø, Development of biomimetic phospholipid vesicle-based permeation assays (PVPA) as screening tool in drug development. Main supervisor Eide Flaten, Co-supervisor Skalko-Basnet
Toril Andersen 2011-2015, University of Tromsø, Novel chitosan-containing liposomes as mucoadhesive delivery system for vaginal administration. Main supervisor Skalko-Basnet, Co-supervisors Eide Flaten, Tho and Mattsson
André Engesland, 2010-2015, University of Tromsø, In vitro permeation models for healthy and compromised skin: The Phospholipid Vesicle-based Permeation Assay (PVPA) for skin applications. Main supervisor Eide Flaten, Co-supervisor Skalko-Basnet
Julia Hurler 2009-2013, University of Tromsø, Improved burns therapy: A formulation development, characterization and optimization of a new liposomes-in-hydrogel system. Main supervisor Skalko-Basnet, co-supervisor Rolf Schubert
Anders Svadberg 2012 Nucleophilic Substitution ReACTIONS FOR Positron Emission Tomography; Factors Influencing the Reactivity od 18F Fluoride. Main supervisor Hjelstuen
Massimiliano Pio di Cagno, 2012, University of Southern Denmark, Odense, Denmark: Overcoming poor solubility and instability of new anticancer drug candidates, Design of water-based formulations for in vitro studies. Main supervisor Bauer Brandl, Co-supervisors Brandl and Skalko-Basnet
Linda Salbu 2011, University of Tromsø, Compressibility and Compactability of Pectin Powders. A study of their potential as direct compression excipients in tablets. Main supervisor Tho, Co-supervisors BauerBrandl
Ingvild Klevan 2011. University of Tromsø, Compression Analysis of Pharmaceutical Powders: Assessment of Mechanical Properties and Tablet Manufacturability Prediction. September 2011, Main supervisor Bauer Brandl, Co-supervisors Alderborn, Uppsala University
Tove Julie Evjen 2011, University of Tromsø, Sonosensitive liposomes for ultrasound-mediated drug delivery, June 2011, Main supervisor Brandl, Co-supervisors Fossheim, Epitarget
Johanna Kanzer 2010, University of Tromsø,. Biopharmaceutical Characterisation of Poorly Water-Soluble Drugs in Solid Dispersions, Main supervisor Brandl, Co-supervisors Tho and Fricker, Heidelberg University, Germany
Rahul Haware 2009, University of Tromsø, Rational approach for evaluation of the compression properties of pharmaceutical excipients: a step towards formulation development tool, Main supervisor Bauer Brandl
Dag Erlend Olberg. 2009, University of Tromsø, Novel [¹⁸F]fluorinated prosthetic groups for the labelling of peptides for positron emission tomography (PET), Main supervisor Hjelstuen, Co-supervisors Brandl
Stefan Hupfeld 2009, University of Tromsø, Size characterisation of liposomes using asymmetrical flow field-flow fractionation: factors influencing fractionation and size determination, Main supervisor Brandl
Daniel Zeiss 2008, University of Tromsø, Ion exchangers in drug delivery : thermodynamic and kinetic aspects of the drug/resin-interaction. Main supervisor Bauer Brandl
Gøril Eide Flaten 2007. University of Tromsø, The phospholipid vesicle-based barrier: a novel method for passive drug permeability screening. Main supervisor Brandl, Co-supervisors Luthman, Gothernburg University, Sweden
Ann Mari Sætern 2004. University of Tromsø, Parenteral liposome- and cyclodextrin formulations of camptothecin. Main supervisor Brandl
Holger Grohganz 2004, University of Tromsø, Vesicular phospholipid gels as a potential implant system for cetrorelix. challenges in development. Main supervisor Brandl
2025
Guro Holm Hauboff: Liposomes for inhalation therapy, Can they deliver to lower respiratory tract? June 2025, Supervisors: Natasa Skalko Basnet (main) and Gøril Eide Flaten
Munam Shahzad: Effect of cationic polymer chitosan on integrity and permeability in the cell-free in vitro permeability model PVPA, June 2025, Supervisors: Gøril Eide Flaten (main) and Marta Mantegna
Stian Trinh: Therapeutic Potential of Vancomycin-Loaded Liposomes – The Antibacterial and Anti-Inflammatory Effects, June 2025, Supervisors: Natasa Skalko-Basnet (main), Lisa Hemmingsen and Terje Vasskog
Madeleine Andersen Solvik: Development of a novel topical nanoformulation for the treatment of atopic dermatitis, June 2025, Supervisors: Maddhusja Nalliah (main), Øystein Grimstad and Natasa Skalko-Basnet
Muhammad Imran: Biopolymers-based functional composite hydrogel for wound healing. June 2025, Supervisors: Ali Raza (main) and Sybil Obuobi
Piarina Reginold: Injectable poloxamer- based in situ gel- forming drug delivery system for periodontitis treatment. June 2025, Supervisors: Ali Raza (main) and Sybil Obuobi
Amanpreet Sing Bhambra: Impact of UV-Sterilization on Nanofibers Containing Chitosan and Soluble Beta-Glucan as Active Wound Healing Ingredients - Method Development, Physical Characterization and Biological Testing. June 2025, Supervisors: Ann Mari Holsæter (main) and Lisa Myrseth Hemmingsen
2024
Marit Melbye Sørensen: Application of bEVs as a bacterial membrane model for interaction studies with NMR, June 2024, Supervisors: Johan Isaksson, Gøril Eide Flaten and Maxim Bril'kov
Brage Hjelle Braa: Exploring the viability of using liposomes and bacterial extracellular vesicles (bEVs) in interaction studies by calorimetric methods, June 2024, Supervisors: Maxim Bril'kov, Gøril Eide Flaten and Johan Isaksson
Charlotte Eilertsen: Optimizing vaginal formulations: Evaluating the correlation between chitosan concentration and mucoadhesiveness, June 2024, Supervisors: Silje Mork and May Wenche Jøraholmen
Marte Kristensen: Liposome-based delivery system for chlorogenic acid - novel methods to evaluate biological activities, May 2024, Supervisors: Natasa Skalko-Basnet (main), Lisa Hemmingsen and Terje Vasskog
Cecilie Thanh Loan Dang: Liposome-based delivery system for quercetin – optimization of delivery system and novel methods to evaluate biological activities, May 2024, Supervisors: Natasa Skalko-Basnet (main), Lisa Hemmingsen and Terje Vasskog
Abina Jeyachanderan: Innføring av nytt IKT-system for medikamentell kreftbehandling ved Sykehusapoteket i Tromsø: En før-studie med fokus på prosesskartlegging og WOMBAT-tidsmålinger, Supervisiors: Ann Mari Holsæter (main), Elin Lehnbom and Else Mari Lensegrav
2023
Victoria Stenbakk: Application of outer membrane vesicles from ESKAPE pathogens as native models of bacterial membranes. Supervisors: Maxim Bril'kov and Gøril Eide Flaten
Agnhild Johansen: Liposomes with Docetaxel and Curcumin – Formulation and in vitro Cytotoxicity.
Supervisors: Ann Mari Holsæter (main), May Wenche Jøraholmen, Synnøve Magnussen og Kjersti Sellæg
Trine Marie Henriksen: Liposomes containing curcumin for the localized therapy of vaginal infections: The effect of surface modification on drug efficacy. Supervisors: May Wenche Jøraholmen (main) and Silje Mork (co-supervisor)
Miranda Ramadani: Liposomal quercetin for localized therapy of vaginal infections: Optimizing mucoadhesive properties. Supervisors: May Wenche Jøraholmen (main) and Silje Mork (co-supervisor)
Tariq Zuboon: Characterization and quantification of protein corona on the surface of liposomes.
Supervisors: Eirik Rustad (main), Natasa Skalko-Basnet (co-supervisor)
Aly Mohamed: Design and optimization of liposomal formulation intended for phagocyte targeting in cancer treatment. Supervisor: Natasa Skalko-Basnet (main), Elena Markova (co-supervisor)
Hassan Ali Alkedro: Lipid Nanoparticle Enabled Co-Delivery of Tetrahedral DNA Framework and Antimicrobial Peptides. Supervisors: Sybil Obuobi (main) and Natasa Skalko-Basnet
2022
Linda Kourng: An Evaluation of Cu(DDC)2-Liposome Production via Dual Centrifugation, Optimization of Production Parameters for Novel Liposomes. Supervisors: Laurens Kersch (University of Freiburg, main) and Natasa Skalko-Basnet (co-supervisor)
Mari Salamonsen: Dual delivery of chlorogenic acid and mimic of antimicrobial peptide – towards wound treatment. Supervisors: Lisa Hemmingsen (main) and Natasa Skalko-Basnet (co-supervisor)
Vegård Borøy: Development of DNA Nanoparticles with Properties for Enhanced Biofilm Uptake. Supervisors: Sybil Obuobi (main), Alexandra de Sousa (co-supervisor) and Natasa Skalko-Basnet (co-supervisor)
Robin Kumar: Characterization of pH-responsive liposomes and the effect of PEGylation and serum on pH-responsiveness. Supervisors: Natasa Skalko-Basnet (main) and Eirik Rustad (co-supervisor)
Pimmat Panchai: Chitosan-modified liposomes for delivery of membrane-active antimicrobials – exploring the role of the polymer. Supervisors: Lisa Hemmingsen (main) and Natasa Skalko-Basnet (co-supervisor)
Cindy Jia Ru Zhang: Process optimization of albumin-stabilized mitotane nanoparticle preparation by dual centrifugation and first lyophilization study. Supervisors: Carolin Langer (University of Freiburg, main) and Natasa Skalko-Basnet (co-supervisor)
2021
Aya Ahmed Tawfeq (2021) Localized therapy of vaginal infections: Liposomes-in-hydrogel delivery system containing curcumin. Supervisor May Wenche Jøraholmen, co-supervisor Natasa Skalko-Basnet
Anna Ngoc Phung (2021) Zwitterionic antimicrobial nanoparticles for biofilm therapy. Supervisor Sybil Obuobi, co-supervisor Natasa Skalko-Basnet
Anjanah Murugaiah (2021) The development of electrospun chloramphenicol containing wound dressing. Supervisors: Ann Mari Holsæter and Laura Victoria Schulte-Werning
2020
Ann Kristin Pettersen: Liposomal formulations for membrane active antimicrobials – Assuring safety through an optimised drug delivery system. Supervisors: Natasa Skalko-Basnet and Lisa Myrseth Hemmingsen
Julie Wik Olaussen: Electrospinning of nanofibers with chitosan and β-glucan as active wound healing ingredients - Optimization and characterization. Supervisors: Ann Mari Holsæter and Laura Victoria Schulte-Werning
Sunniva Brurok: High throughput lipolysis-permeation in vitro model employing the mucus-PVPA-mucus barriers to assess drug absorption from lipid based delivery systems. Supervisors: Margherita Falavigna and Gøril Eide Flaten
Kasi Rashid Shorsh: Liposome and chitosan-based delivery systems for epicatechin: Targeting the local treatment of vaginal infections. Supervisors: May Wenche Jøraholmen and Nataša Škalko-Basnet
Silje Mork: Microfluidized liposomes for cellular uptake imaging: Optimization.
Supervisors: Natasa Skalko-Basnet and Jennifer Cauzzo
Luquman Ahsan: Development of chitosan-based delivery system for membrane active antimicrobials. Supervisors: Natasa Skalko-Basnet and Lisa Myrseth Hemmingsen
2019
Eirik André Lindeløff Rustad: Evaluation and late-stage fluorinate of small molecule GnRH-R antagonists. Supervisors: richard Fjellaksel, Ole Kristian Hjelstuen and Jørn H. Hansen
Sonali Bala: Osmotically-active liposomes for nasal drug delivery. Supervisors: Iren Yeeling Wu and Massimiliano di Cagno
Abhilasha Bhargava: Chitosan-based mucoadhesive drug delivery systems for vaginal infections. Supervisor Natasa Skalko-Basnet, co-supervisor May Wenche Jøraholmen
2018
Eivind Gagnat: Improved chronic wound therapy: surface charged deformable liposomes for dermal delivery of curcumin. Supervisor Selenia Ternullo, co-supervisor Natasa Skalko-Basnet
Mia Tostrup: Liposomes-in-hydrogel delivery system containing resveratrol for the local treatment of vaginal infections. Supervisor May Wenche Jøraholmen, co-supervisor Natasa Skalko-Basnet
Sabrin Moueffaq: Topical vaginal therapy: Development of a liposome-in-hydrogel delivery system for epicatechin. Supervisor May Wenche Jøraholmen, co-supervisor Natasa Skalko-Basnet
Nicklas Ekblad: Melt processability of amorphous solid dispersions during hot-melt extrusion. Supervisor Jukka Rantanen (University of Copenhagen), co-supervisor Natasa Skalko-Basnet
Iselin Karlsen: Docetaxel in liposomes: the effect of lipid composition on the achieved drug entrapment. Supervisor Ann Mari Holsæter, co-supervisor Natasa Skalko-Basnet
Trygg Einar Nikolaisen: Influence of environmental tonicity changes on lipophilic drug release from liposomes. Supervisor Massimiliano Pio di Cagno, co-supervisor Iren Yeeling Wu
Nikolay Fadeev: B-cyclodextrin polymers as cholesterol sequestrating agents. Supervisor Massimiliano Pio di Cagno
2017
Christoffer J. Løkse: Synthesis of copper and silver nanoparticles with extracts of berries: A green chemistry approach. Supervisor Sandra Ristori, University of Florence and co-supervisor Natasa Skalko-Basnet, UiT
2016
Awfa Al-Shamkawy: Interaction of CPZEN-45, a novel anti-tubercular drug candidate, with immune cells in vitro. Supervisor Carsten Ehrhardt, Trinity College Dublin, co-supervisor Gøril Eide Flaten, UiT
Lloyd Mbugua Kangu: Chitosan lecithin nanoparticles with New Chemical Entity, Nanotoxicity evaluation. Supervisor Natasa Skalko-Basnet, UiT and co-supervisor Gry Stensrud, Photocure
Damir Dugalic: Assessment of Anchimerically-Assisted Finkelstein Reaction of Application in Late-Stage Radioiodination. Supervisor Ole Kristian Hjelstuen UiT, co-supervisors Jørn Hansen UiT and Richard Fjellaksel UiT/UNN
2015
Ayantu Edossa Chemeda: Topical liposomes treted by probe-sonication: Study on process and composition using statistical experimental design and multivariate evaluation. Supervisor Ann Mari Holsæter, co-supervisor Sveinung Gaarden Ingebrigtsen
Hege-Kristine Lorentzen: Konsekvensene av deling og knusing av enteroformuleringer - Metodeutvikling og evaluering. Supervisor Daniel Zeiss (HiNT), co-supervisor Gøril Eide Flaten
Iren Yeeling Wu: Can nanomedicine improve the semen quality? The potentials of liposomal curcumin. Supervisor Purusotam Basnet (UNN, IKM), co-supervisor Natasa Skalko-Basnet
Lisa Myrseth Hemmingsen: Chitosan lecithin nanoparticles with New Chemical Entity, Antimicrobial evaluation. Supervisor Natasa Skalko-Basnet, co-supervisor Gry Stensrud (Photocure)
Evrard Louis A B Betoko: Development of orodispersable films: A new age-appropriate dosage form for children. Supervisor Ingunn Tho (University of Oslo), co-supervisor Natasa Skalko-Basnet
Irja Alainezhad Kjærvik:Electrospun amphiphilic nanofibers for the in situ preparation and delivery of drug-loaded liposomes. Supervisors Jyrki Heinämäki, Karin Kogermann, Ivo Laidmäe (University of Tartu, Estonia), co-supervisor Natasa Skalko-Basnet
Kristina Rybak: Mucus-penetrating drug carriers for vaginal drug delivery. Supervisor May Wenche Jøraholmen, co-supervisor Natasa Skalko-Basnet
Joseph Azumah: Liposomes as potential carrier for bioactive β2,2-amino acid derivatives A feasibility study. Supervisors Mørten Strøm, Dominik Ausbacher, co-supervisor Natasa Skalko-Basnet
2014
Anna N. Troyan: Novel Liposome Formulation from algae membranes as carriers for bioactive compounds (supervisor Sandra Ristori (University of Florence, Italy), co-supervisor Natasa Skalko-Basnet)
Marthe Karoline Grønvold: Coating of neutral liposomes with hydrophobically modified hydroxyethyl cellulose (supervisor Gro Smistad (UiO), co-supervisor Natasa Skalko-Basnet)
Muna Abukar Hadafow: Chitosan-based drug delivery system for a chemical entity for photodynamic wound therapy (supervisors Natasa Skalko-Basnet and Gry Stensrud (Photocure ASA))
Ida Emilie Thoresen: Advanced drug delivery system for new chemical entity destined for wound therapy: Anti-biofilm potential of novel drug delivery system(supervisors Natasa Skalko-Basnet and Gry Stensrud (Photocure ASA))
Malin Nyland Aalberg: Mukus-penetrerende legemiddelbærere: Hvordan modifisering av overflaten påvirker terapeutisk effect (supervisors Natasa Skalko-Basnet and May Wenche Jøraholmen)
Kurt Jonny Johansen: Undersøkelse av en ny topikal antimykotisk “liposom-i-hydrogel” legemiddelformulering med virkestoffet flukonazol (supervisors Ann Mari Holsæter and Sveinung G. Ingebrigtsen)
Herra Iqbal: Kompatibilitetstesting av TPN og legemidler gitt som Y-infusjon til barn (supervisors Ingunn Tho and Vigdis Staven)
2013
Eirik Hagen. Orally disintegrating mini-tablets for children: Using interactive mixtures to obtain mini-tablets of high dose-homogeneity (Supervisor Ingunn Tho, co-supervisor Sofia Mattsson (Umeå University))
Rebwar Salar Nori Saleh. Development of mucoadhesive polymer-caoted liposomes for hydration of the oral mucosa (Supervisor Gro Smistad(UiO), co-supervisors Marianne Hiorth (UiO) and Natasa Skalko-Basnet)
Samia Riaz. Limitations of pig skin as in vitro model mimicking skin with damaged barrier properties(G.E. Flaten, N. Skalko-Basnet, A. Engesland)
Elena Fedreheim. Development of an in vitro model to study compromised skin: pigskin versus the phospholipid vesicle-based permeation assay (G.E. Flaten, N. Skalko-Basnet, A. Engesland)
2012
May Wenche Bakke Jøraholmen. Development of chitosan-coated liposomes for improved therapy of vaginal infections: clotrimazol as model drug (N. Skalko-Basnet, I. Tho)
Else Mari Ødegaard-Jensen. Liposomes as drug delivery system in mitochondrial targeting
(N. Skalko-Basnet, R. Süss (University of Freiburg, Germany))
Truc Phuong Nguyen. Optimization of antimicrobial wound dressings: Liposomal hydrogels with mupirocin (N. Skalko-Basnet, J. Hurler)
Sveinung Gaarden Ingebrigtsen. Development of targeted liposomal drug delivery vehicle (G.E. Flaten, R. Whitaker)
Johanne Naper Trønnes. Development of liposomal formulation for green tea catechins targeted for the treatment of vaginal inflammation. (N. Skalko-Basnet, P. Basnet)
2011
Kristin Aasarød. Metodeutvikling og innledende kompatibilitetsstudie mellom antiinfektiva og Olimel N5E gjennom Y-sett infusjon til barn fra 10 - 50 kg (I. Tho, N. Skalko-Basnet)
Vigdis Staven. Kompatibilitetsstudie mellom legemidler og TPN gitt som parallellinfusjon til pediatriske pasienter – metodeutvikling og innledende tester med fem ulike legemidler og én total parenteral ernæringspose (I. Tho, N. Skalko-Basnet)
Elenaz Naderkhani. Investigation and optimization of liposome formulations for use as drug carrier for the anticancer agent Camptothecin (G.E. Flaten, R. Whitaker)
Ole Aleksander Berg. Advanced delivery systems for skin and burns thersapy: Mupirocin as an antibacterial model drug (N. Skalko-Basnet, J. Hurler)
Hilde-Gunn Meland. Development of mucoadhesive MUDS for improved vaginal therapy (I. Tho, N. Skalko-Basnet)
Fredrik Sandberg Løding. Use of ordered mixtures to obtain high dose homogeneity in mini-tablets: Studies of orally disintegrating systems for children (I. Tho, S. Mattsson (Umeå University, Sweden))
2010
Haider Hussain. Development of liposomal curcumin for vaginal drug delivery (N. Skalko-Basnet and I. Tho)
Bahador Poorahmary Kermany. Carbopol hydrogels for topical administration: treatment of wounds (N. Skalko-Basnet)
André Engesland. Hydrogels of natural origin in wound healing: Formulation development (N. Skalko-Basnet)
2009
Susanne Nilssen. Utvikling av en oral formulering baset på minitableter for bruk til diagnostisering av tykktarmskreft. (I. Tho).
Judit Chipo Tendeland. Karakterisering og optmalisering av myke omega-3-kapsler med kalsium. (I. Tho)
Tormund Møkleby. Active loading of gemcitabine into liposomes. (M. Brandl; U. Massing(Tumor Bio, Freiburg, Germany))
2008
Vincent Ruca Kagabo. Studie av tørking av myke gelatinkapsler med varierende filmtykkelse. Et prosjekt i samarbeid med ProBio AS. (I. Tho)
Helene Moen. Influence of osmotic stress on liposome size and morphology. (S. Hupfeld; M. Brandl)
Julia Hurler. Ion exchange for drug delivery: Drug binding of propranolol HCl to polymer sulfonates of different shape and structure. (A. Bauer-Brandl)
2007
Skjalg Nyheim Solum. Komprimeringsegenskaper hos minitabletter sammenlignet med vanlige tabletter (A. Bauer-Brandl; I. Tho)
Lene Holst. Myke Gelatinekapsler; en studie av tørkebetingelser og egenskaper. (I. Tho)
Ateh Nkem Atabong. Development and Validation of a non- destructive method for quantification of the active ingredient and rest water content in Metformin Hydrochloride granules based on Near Infrared Spectroscopy (NIRS). (I. Tho, B. Sæten (Weifa))
Mari K.S. Lawrence. Development of small scale production of pH-sensitive liposomes. (M. Brandl, U. Massing (Tumor Bio, Freiburg, Germany))
Berit K.S. Solvik. A novel recombinant protein promoting cell adhesion for use in tissue engineering. (M. Brandl ; C. van der Walle (University of Strathclyde))
Tove Julie Evjen. Development of improved bendamustin-liposomes. (M. Brandl; U. Massing (Tumor Bio, Freiburg, Germany))
Opeyemi Awoyemi. Transfer of the phospholipid vesicle-based drug permeability assay to an automated laboratory handling system.(M. Brandl, G. E. Flaten, U. Massing (Tumor Bio, Freiburg, Germany))
Sarah Fischer. Ion exchange for drug delivery: Drug binding of Propranolol HCl to polystyrene and dextran sulfonates (R. Schubert, A. Bauer-Brandl)
Dominik Ausbacher. A4F/MALS-analysis of liposomes: influence of key factors on fractionation behavior and evaluation of MALS fit routins. (S. Hupfeld; M. Brandl)
2006
Hilde Gravem. Gemcitabine containing liposomes. (M. Brandl)
Nina B. Brynjulfsen. Potential utility of the reactarray workstation for the rapid development of novel 18-F fluoride radiolabelled compounds. (O.K. Hjelstuen; N.Osborn (GE Healthcare, Amersham))
Kine H. Brækkan. Formulation and purification of a radiolabelled compound for imaging of Alzheimer's disease. (O.K. Hjelstuen; R)
2005
Therese Riis. Development of extended release formulations (mini matrix tablets) for different drug substances by using Kollidon® SR (A. Bauer-Brandl)
Ingvild Klevan. Evaluation of compression parameters in the Shapiro equation. (A. Bauer-Brandl)
2004
Morten Herman Vogt. Water granulation of hydrophillic matrix polymers. (A. Bauer-Brandl; A. Larsson (Chalmers, Göteborg))
2003
Jan Erik Olsen. AmoLac-a new excipient for tableting (A. Bauer-Brandl)
Simon B. Jensen. Effect of amorphous content on the compactability of lactose. (A. Bauer-Brandl)
Gøril Eide Flaten. Incorporation of camptothecin in liposomes, method development and incorporation efficacy screening using different liposome formulations. (M. Brandl; A. Sætern)
Nguyen Binh Nguyen. Effekt av pH og hydroksypropyl-beta-cyclodekstrin på løselighet av camptothecin og dens lakton-karboksylat likevekt; en faseoppløselighetsstudie. (M. Brandl; A. Sætern)
Christer Bakke Frantzen. Studies on the size distribution of submicron particles using Photon Correlation Spectroscopy and Size Exclusion Chromatography. (M. Brandl; U. Massing (Tumor Bio, Freiburg, Germany))
Nina Pettersen. Wetting and swelling behaviour of novel film-coating materials for colon drug targeting. (A. Bauer-Brandl)
Kjetil Bjørkli.Ekstraksjon og rensing av marine fosfolipider: farmasøytiske og nutrasøytiske anvendelser (E. Løvaas; M. Brandl; H. Grohganz; M. Moe)
2002
Åsmund Braaten. Bestemmelse av fosfolipidhydrolyse i liposomer med cellegiften camptothecin ved hjelp av tynnsjiktsanalyse. (M. Brandl; A. Sætern)
Gro Dahlseng Håkonsen. Development and validation of a high performance liquid chromatographic assay for the determination of gemcitabine (dFdC) and its metabolite dFdU in human plasma. (M. Brandl; U. Massing (Tumor Bio, Freiburg, Germany))
2001
Hassan Tarraf. Performance of virus inactivation procedures in horse serum: recombinant adenovirus (AdLox) as a potential tool (O. Schläfli)
Lars Ingebrigtsen. Size analysis of submicron particles and liposomes by size exclusion chromatography and photon correlation spectroscopy (M. Brandl)
2000
Liv Johanne Sætern. En pilotstudie for opprettelse av en liposomemodell for studier av legemiddelsubstansers evne til passiv absorpsjon gjennom biologiske membraner. (M. Brandl; K. Luthman)
Beate Løseth Tverberg. Vesicular phospholipid gels as implantable depot systems: influence of lipid composition on in vitro release behaviour (M. Brandl)
Anne Cecilie Larsen. Liposome production by detergent removal using saturated phospholipids: feasibility & formulations variables. (M.Brandl; GE Healtcare)
1999
Ann Mari Sætern. Utvikling av et preparasjonssett for merking av antisens oligonukleotider med Technetium-99m. (O.K. Hjelstuen)
Truls Bjørknes. Stabilitet av petidin injeksjonsvæske i plastampullar (P. Waaler)