Drug delivery for advanced skin therapy
 Summary of approaches used to delivery efficient localized therapy of skin disorders
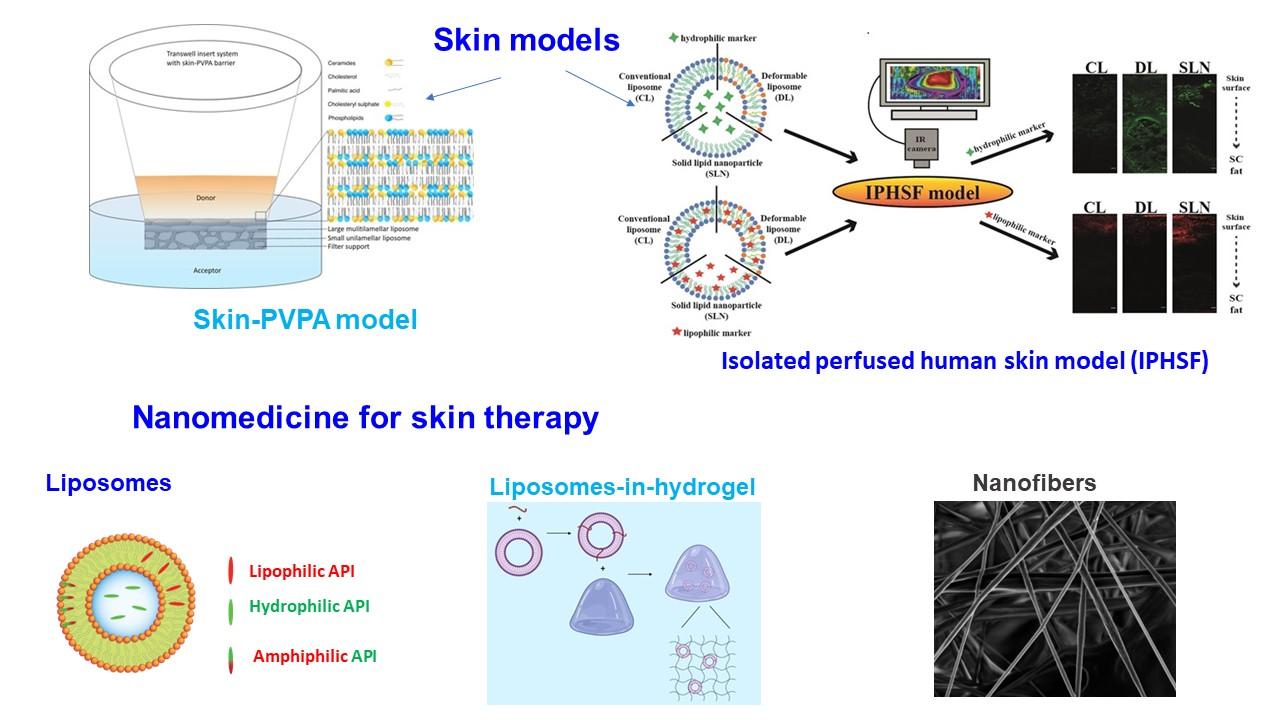
Summary of approaches used to delivery efficient localized therapy of skin disordersDue to its unique nature, skin can be seen as both a very challanging barrier that drug or active ingredients will face in (trans)dermal delivery as well as the site that offers numerous options for smart delivery both dermally as well as transdermally. To address its complexity, we have developed skin-mimicking in vitro models able to assist in optimization of various formulations destined for skin administration. Moreover, to come closer to in vivo conditions yet avoiding use of animals, we also developed ex vivo perfused human skin flap model that permits following the fate of nanocarrier on/in skin for up to 6 hours, thereof enabaling deeper insight on the interplay between skin and nanocarriers that assist in optimization of formulation. In recent years, our focus has been on design and development of superior therapy for skin infections and inflammatory disorders, particularly considering rising treat of antimicrobial resistance. The therapy relies on advanced nanocarriers, nanocarriers-in-hydrogels formulations as well as nanofibers. Moreover, we try to use natural origin active and pharmaceutical ingredients and recycled drugs.
The whole project is diveded into several sub-projects that are both interconnected and independent in respective pipelines and principal investigators (PIs).
a) skin-mimicking PVPA in vitro model (PI Eide Flaten): Focusing on development of simple and affordable skin models for transdermal permeation of drug candidates and evaluation of various drugs and formulations at an early development stage in order to avoid excessive use of animal experiment.
b) human skin perfused ex vivo model (PI Skalko-Basnet): The isolated perfused human skin flap that remains metabolically active tissue for up to 6 hours during in vitro perfusion was developedas a close-to-in vivo skin penetration model. The validated model can be applied in optimization of various formulations destined for skin administration.
c) peptidomimetics as novel approach to target biofilms (PI Skalko-Basnet): Advanced topical delivery systems for membrane-active antimicrobials that are novel synthetic mimics of antimicrobial peptides developed at UiT The Arctic University of Norway, in collaboration with Dr. Lisa Hemmingsen and Prof. Morten Strøm. Novel liposomes-in-chitosan-hydrogels formulations are challanges against bacteria and biofilms to assist in treatment of acute and chronic wound infections.
d) multifunctional wound dressings as nanofibers or hydrogels (PI Holsæter): Aims at improving local wound therapy trough utilizing materials with promising biological activity, as well as exploring the different format's ability to support wound healing by aligning with the bodies natural healing mechanisms. We also focus on promise of up-scaling, sustainability and personalized medicine by optimizing techniques such as wire-electrospinning and the dual centrifugation.
e) atopic dermatitis
-
Nanomedicine for efficient localized treatment of atopic dermatitis (PI Skalko-Basnet): development of multipurpose advanced formulations for patient-friendly therapy
-
Targeting inflammation in atopic dermatitis using nanomedicine-based localized topical therapy (PI Grimstad): targeting inflammation pathways via novel delivery systems for effieicnt topical therapy
f) Coalition (PI Obuobi)
Collaborating networks:
NANESC
Netskinmodel
We appreciate continuous support of our international collaborators:
Professor Zeljka Vanic, University of Zagreb, Croatia
Professor Barbara Luppi and Dr. Barbara Giodani , University of Bologna, Italy
Professor Fabio Sonvico, University of Parma, Italy
Professor Francesco Cilurzo and Dr. Silvia Franze, University of Milan, Italy
Selected publications:
A. Sousa, A.N. Phung, N. Škalko-Basnet and S. Obuobi (2023)
Smart Delivery Systems for Microbial Biofilm Therapy: Carrier Design, Drug Release and Toxicology. Journal of Controlled Release, https://doi.org/10.1039/d3tb00704a
L.M. Hemmingsen, B. Giordani, M.H. Paulsen, Ž. Vanić, G.E. Flaten, B. Vitali, P. Basnet, A. Bayer, M.B. Strøm and N. Škalko-Basnet (2023)
Tailored anti-biofilm activity – liposomal delivery for mimic of small antimicrobial peptide. Biomaterials Advances, https://doi.org/10.1016/j.bioadv.2022.213238 (Journal is formerly known as Materials Science and Engineering: C
L.M. Hemmingsen, P. Panchai, K. Julin, P. Basnet, M. Nystad, M. Johannessen and N. Škalko-Basnet (2022)
Chitosan-based delivery system enhances antimicrobial activity of chlorhexidine. Frontiers in Microbiology, section Infectious Agents and Disease, https://doi.org/10.3389/fmicb.2022.1023083
S. Obuobi, A.N. Phung, K. Julin, M. Johannessen and N. Škalko-Basnet (2022)
Biofilm Responsive Zwitterionic Antimicrobial Nanoparticles to Treat Cutaneous Infection. Biomacromolecules, https://doi.org/10.1021/acs.biomac.1c01274
J. Grip, E. Steene, R.E. Engstad, J. Hart, A. Bell, I. Skjæveland, P. Basnet, N. Škalko-Basnet, and A.M. Holsæter (2021)
Development of a novel beta-glucan supplemented hydrogel spray formulation and wound healing efficacy in a db/db diabetic mouse model. European Journal of Pharmaceutics and Biopharmaceutics, https://doi.org/10.1016/j.ejpb.2021.10.013
L.V. Schulte-Werning, A. Murugaiah, B. Singh, M. Johannessen, R.E. Engstad, N. Škalko-Basnet and A.M. Holsæter (2021)
Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-inflammatory Properties prepared by Needle-free Electrospinning. Pharmaceutics, https://doi.org/10.3390/pharmaceutics13091527
G.E. Flaten, Ž. Vanic, N. Škalko-Basnet (2021)
The Role of In Vitro Skin Models in Optimization of Dermal Drug Delivery, in N.Dragićević and H. Maibach (eds), Percutaneous Absorption, Fifth edition, Taylor and Francis, 693-714
L.M. Hemmingsen, B. Giordani, A.K. Pettersen, B. Vitali, P. Basnet and N. Škalko-Basnet (2021)
Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohydrate Polymers, https://doi.org/10.1016/j.carbpol.2021.117939
S. Obuobi, K. Julin, E.G. Fredheim, M. Johannessen and N. Škalko-Basnet (2020)
Liposomal delivery of antibiotic loaded nucleic acid nanogels with enhanced drug loading and synergistic anti-inflammatory activity against S. aureus intracellular infections. Journal of Controlled Release, https://doi.org/10.1016/j.jconrel.2020.06.002
Members:
Financial/grant information:
The Norwegian Animal Protection Alliance funding for our work on reduced use of animals in research (2012; 2021) and the Norecopa’s 3R prize in 2013 for our work.
The Research Council of Norway (grant number 240123/O30; 2014-2017); Industrial PhD project of Jostein Grip, Biotec BetaGlucans AS. UiT The Arctic University of Norway
Thematic strategic priorities UiT funding
“Lead-to-drug development of amphipatic scaffolds targeting multi-resistant bacteria” (project no. 235569; 2018-2022).
“Advanced method for determining metabolic activity in bacteria and cells for increased knowledge in the fight against resistant bacteria (CalScreener)” (infrastructure funding)