Cell free in vitro permeability models
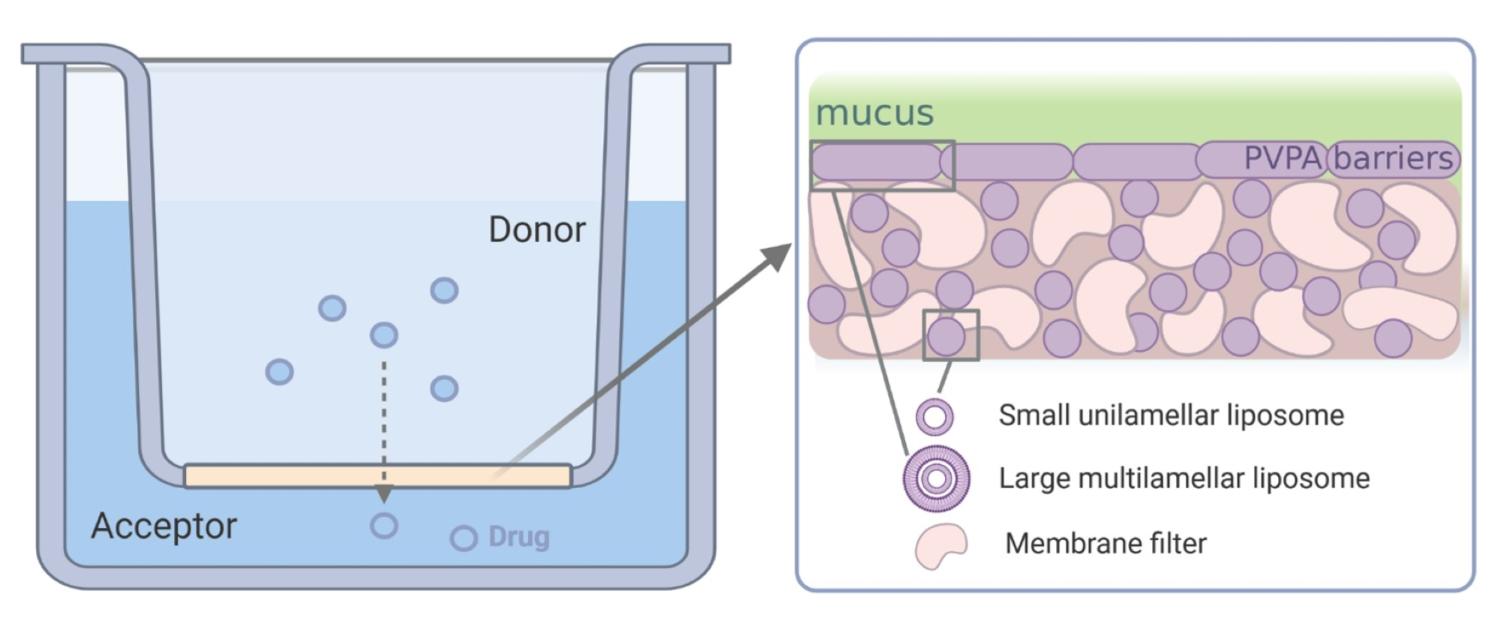 A schematic illustration of the structure of the PVPA barrier and set up. The pink is the filter support while the purple represent the liposomes found both inside the pores as well as in a layer on top of the filter, and the green representing the mucus layer (prepared with Biorender by Margherita Falavigna)
A schematic illustration of the structure of the PVPA barrier and set up. The pink is the filter support while the purple represent the liposomes found both inside the pores as well as in a layer on top of the filter, and the green representing the mucus layer (prepared with Biorender by Margherita Falavigna) Permeability of compounds through membranes is of great interest and importance for elucidation of many biological cell functions. The majority of the metabolically important substances are transported across membranes by active transport, but many other intrinsic compounds as well as the majority of drugs are known to pass the membrane by passive diffusion. For a drug substance to act systemically after administration if has to overcome different biological barriers to reach the point of action, which is why our research group focuses on passive permeability prosesses of drug through different membranes.
The phospholipid vesicle-based permeation assay (PVPA), has been developed in our group and its suitability for measurement of passive drug permeability has been demonstrated. The barrier consists of a tight layer of liposomes on a filter support which is produced by first depositing smaller liposomes meant to go into the pores and then larger liposomes that will layer on top of the filter, by use of centrifugation. Freeze-thaw cycling is then used to promote liposome fusion to generate a tight barrier. The characterization of the barrier structure showed that the pores of the filter are filled with liposomes and that there is a layer of liposomes on top of the filter (figure above). The model was rapidly included among the well-established models for permeability estimation after the first introduced in 2006 appearing in the first review already in 2007.
The lipid composition of the lipsomes as well as the environment on the donor side can be adjusted to mimick different barrers in the body. In our research we are focusing in the intestinal, vaginal and skin barrier.
A. Intestinal barrier
Oral administration of drugs is preferred over any other administration route due to the convenience for the patients and the resulting good compliance. An important property of a drug is its bioavailability, and for an orally administered drug the bioavailability is mainly limited by its solubility and/or permeability through the intestinal epithelia. It is therefore important to have access to methods suitable for rapid screening of the permeability properties of large numbers of new drug candidates early in drug development.
The permeability values obtained from the phospholipid vesicle-based model for a diverse set of compounds correlate well with literature data on in vivo absorption of the drugs in humans. By using the common classification system for permeability properties 80% of the initially tested compounds were correctly classified. These studies showed that our approach appears to model the in vivo absorption equally well or even better than the DS-PAMPA model and equally well as the Caco-2 cell model. In addition, it is a lot easier to handle than the cell based assays. The PVPA has further been used to successfully evaluate the performance of different formulations in formulation optimization studies. The model has also shown to be prone for automation using a laboratory robotic system.
The latest addition to the family of the intestinal PVPA is the Mucus-PVPA. The mucus-PVPA consists of a PVPA barrier covered with mucus to more closely mimic the in vivo situation where the mucus is making up an important additional barrier to the epithelial cells. Different types of mucus has also been tested, and promising results conserning formulation optimization has been obtained. The Mucus-PVPA has also been sucsessfully combined with a lipolysis setup to enable estimation of the in vivo absorbtion behaviour of lipid based systems.
B. Vaginal barrier
To optimize formulations destined for the vaginal site, it is important to evaluate the drug retention within the vagina as well as its permeation across the mucosa, particularly in the presence of vaginal fluids (SVF) as well as mucus. While developing the vaginal-PVPA in vitro permeability model we showed that both presence of mucus, SVF as well as the pH in the fluide is of importancel to better understand and predict the in vivo performance of formulations destined for vaginal administration. The vaginal-PVPA in vitro permeability model has been established and validated as a tool to evaluate both the permeation of drugs as wells as drugs from different formulatioens e.g. liposomal formulations.
C. Skin barrier
The development of simple and affordable skin models for transdermal permeation of drug candidates and evaluation of various drugs and formulations at an early development stage is also of high impostance. Most of the current methods used in investigation of topical administration and treatment of damaged skin rely on animal models. Our goal was therefore to develop an in vitro model for both healthy and compromized skin in order to avoid excessive use of animals and human models in early phase development of topical formulations and active substances intended for topical administration.
When developing the skin-PVPA changes in lipid composition of the liposomes used to produce the lipid-based barrier was done to better mimic the in vivo stratum corneum lipid composition. This required optimization of manufacuturing conditions applied in barrier formation and we were also able to use modification in the prparation prosess to prepare barriers of incremental degree of leakiness, representing different degree of intact and compromized skin. The skin-PVPA models appears to be able to distinguish between drugs with different degree of lipophilicity and penetration potential as well as be used in evaluations of formulations intended for skin administration. The model could therefore be applied in both pharmaceutical and cosmeceutical manufacturing and also has potential to provide deeper insight on safety of nanodelivery systems administered onto the skin.
Collaborating consortia:
NANESC (Non-animal dependent research cluster in Tromsø)
UNGAP
NetSkinModel (Cost action)
Nordic POP
Selected publications
- P. Rainsford, R.B. Sarre, B.O. Brandsdal, M. Falavigna, G.E. Flaten, M. Jakubec and J. Isaksson (2022), WIND-PVPA: Water/Ion NMR Detected PVPA to assess lipid barrier integrity in vitro through quantification of passive water- and ion transport, BBA – Biomembranes, accepted
- M. Šoltys, D. Zůza, T. Boleslavská, S. Akhlasová, M. Balouch, P. Kovačík, J. Beránek, N. Škalko-Basnet, G.E. Flaten and F. Štěpánek (2021) Drug loading to mesoporous silica carriers by solvent evaporation: A comparative study of amorphization capacity and release kinetics, International Journal of Pharmaceutics, 607, 120982
- M. Falavigna, S. Brurok, M. Klitgaard, G.E. Flaten (2021) Simultaneous assessment of in vitro lipolysis and permeation in the mucus-PVPA model to predict oral absorption of a poorly water soluble drug in SNEDDSs, Int J Pharm, 596,12025, DOI:https://doi.org/10.1016/j.ijpharm.2021.120258
- M. Falavigna, M. Klitgaard, R. Berthelsen, A. Müllertz, G.E. Flaten (2021) Predicting oral absorption of fenofibrate in lipid-based drug delivery systems by combining in vitro lipolysis with the mucus-PVPA permeability model, JPharmSci, 110, 208-216, DOI:https://doi.org/10.1016/j.xphs.2020.08.02
- M. Falavigna, M. Pattacini, R. Wibel, F. Sonvico, N.Škalko-Basnet, G.E. Flaten (2020), The vaginal-PVPA: a vaginal mucosa-mimicking in vitro permeation tool for evaluation of mucoadhesive formulations, Pharmaceutics, 12, 568
- M. Falavigna, M. Klitgaard, E. Steene and G. E. Flaten (2019) Mimicking regional and fasted/fed state conditions in the intestine with the mucus-PVPA in vitro model: the impact of pH and simulated intestinal fluids on drug permeability, European Journal of Pharmaceutical Sciences, 132, 44-54
- P. Berben, A. Bauer-Brandl, M. Brandl, B. Faller, G. E. Flaten, A. Jacobsen, J. Brouwers and P. Augustijns (2018), Drug Permeability Profiling using Cell-Free Permeation Tools: An Overview and Their Applications, EurJPharmSci, 119, p 219-233
- M. Falavigna, M. Klitgaard, C. Brase, S. Ternullo, N. Škalko-Basnet, G. E. Flaten (2018), Mucus-PVPA (Mucus Phospholipid Vesicle-based Permeation Assay): an artificial permeability tool for drug screening and formulation development, International Journal of Pharmacutis, 537.(1-2) s. 213-222
- E. Naderkhani, T. Vasskog, G.E. Flaten (2015) Biomimetic PVPA in vitro model for estimation of the intestinal drug permeability using fasted and fed state simulated intestinal fluids, European Journal of Pharmaceutical Sciences, 73, 64-7
- G.E Flaten, Z. Palac, A.Engesland, J. Filipović-Grčić, Ž. Vanić and N. Škalko-Basnet (2015), In vitro skin models as a tool in optimization of drug formulation, European Journal of Pharmaceutical Sciences, 75, 10–24
- A. Engesland, N. Škalko-Basnet, G. E. Flaten (2015), PVPA and EpiSkin® in Assessment of Drug Therapies Destined for Skin Administration, Journal of Pharmaceutical Sciences, 104:1119–1127
- E. Naderkhani, A. Erber, N. Škalko-Basnet, G.E. Flaten (2014), Improved permeability of acyclovir: Optimization of mucoadhesive liposomes using the PVPA model, Journal of Pharmaceutical Sciences, 103, 661-668
- Z. Palac, A. Engesland, G.E. Flaten, N. Škalko-Basnet, J. Filipović-Grčić, Ž. Vanić (2014), Liposomes for (trans)dermal drug delivery: the skin-PVPA as a novel in vitro stratum corneum model in formulation development, Journal of Liposome Research, accepted
- E. Naderkhani , J. Isaksson, A. Ryzakov, G.E. Flaten (2014), Development of a biomimetic phospholipid vesicle-based permeation assay (PVPA) for the estimation of intestinal drug permeability, Journal of Pharmaceutical Sciences, accepted
- Engesland, A.; Skar, M.; Hansen, T.; Skalko-Basnet, N.; Flaten, G. E. (2013), New Applications of PVPA: Permeation Model Mimicking Skin Barrier, Journal of Pharmaceutical Sciences, 102:1588-1600
- Flaten, Gøril Eide; Gabor, Kottra; Stensen, Wenche; Isaksen, Geir; Karstad, Rasmus; Svendsen, John S; Daniel, Hannelore and Svenson, Johan (2021), In vitro characterisation of human peptide transporter hPEPT1 interactions and passive permeation studies of short cationic antimicrobial peptides, J Med Chem (2011) 2422-2432
- Kanzer, J; Tho,I; Flaten, G.E; Maegerlein,M; Hoelig,P; G. Fricker, G; Brandl, M (2010), In-vitro permeability screening of melt extrudate formulations containing poorly water-soluble drug compounds using the phospholipid veicle-based barrier, Journal of Pharmacy and Pharmacology 2010, 62: 1591–1598
- Flaten, Gøril Eide; Luthman, Kristina; Vasskog, Terje; Brandl, Martin (2008), Drug permeability across a phospholipid vesicle-based barrier: 4. The effect of tensides, co-solvent and pH changes on barrier integrity and on drug permeability. European Journal of Pharmaceutical Sciences, 34.(2-3) s. 173-180
- Flaten, Gøril Eide; Dhanikula, Anand Babu; Luthman, Kristina; Brandl, Martin (2006), Drug permeability across a phospholipid vesicle based barrier: A novel approach for studying passive diffusion. European Journal of Pharmaceutical Sciences, 27.(1) s. 80-90
Members:
Financial/grant information:
Mohn fondation
CANS
The Norwegian Animal Protection Alliance
NordicPOP
UNGAP