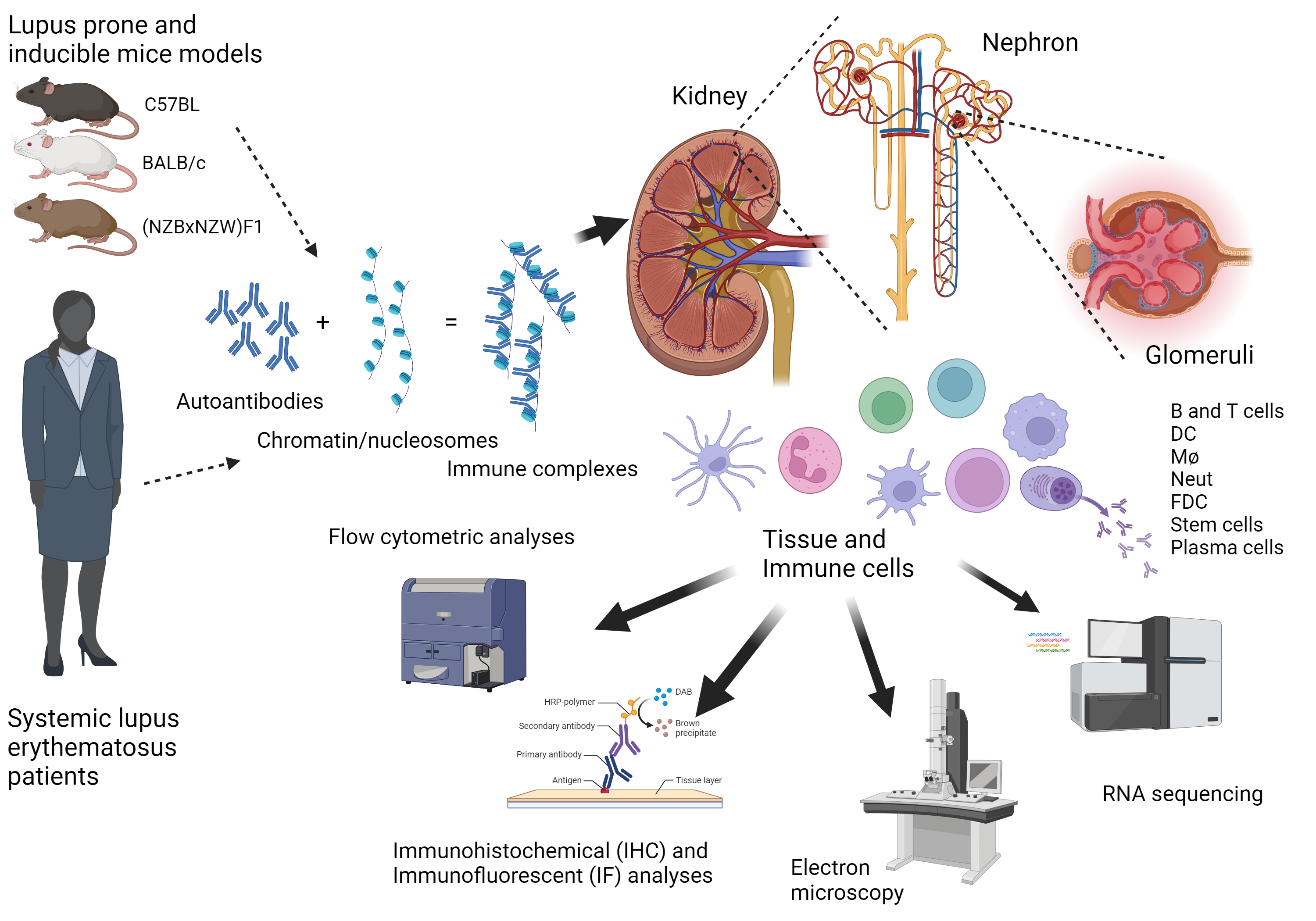
Tertiary lymphoide structures in autoimmunity
Several diseases like systemic lupus erythematosus (SLE), diabetes mellitus (DM), Sjøgren’s Syndrome (SS), IgA nephropathy (IgAN), membranous glomerulonephritis (MGN), interstitial nephritis (IN), hypertensive nephrosclerosis (HN) and renal cancers may lead to the development of chronic kidney disease (CKD). There is a need for early disease detection to start treatment at a point where the tissue destruction due to inflammation is low. A common factor between different inflammatory kidney diseases may be the development of tertiary lymphoid structures (TLS). If inflammatory kidney damage is not detected early enough, the patient may have an increased risk of developing end stage kidney disease (ESKD) that requires kidney replacement therapy (dialysis or kidney transplantation). Extensive efforts are needed to fill the knowledge gaps regarding the complexity of molecular disease mechanisms, diagnostic procedures, and treatment development. We aim to develop new clinical imaging techniques to diagnose and monitor disease development, prevent ESKD, and improve patient management.
TLS formation refers to the development of organized lymphoid structures which resemble secondary lymphoid organs (spleen, lymph nodes (LyN) and payers patches (PPs)) in tissues that are targeted by chronic inflammatory processes, such as infection and autoimmunity1, 2, 3. The formation of these structures and their association with tissue destruction suggest that they are important inductive sites for self-reactive T, B and plasma cells producing autoantibodies that contribute to an autoimmune disease process4. However, since the structures resembles secondary lymphoid organs, they could also be an important site for local immune regulation. Indeed, in atherosclerosis, formation of TLS has been shown to be protective5, and in cancer, induction of TLS by the cancer cells induce immunologic tolerance towards the tumor6. The development of TLS has been observed in many autoimmune diseases like MS7, DM8 and SS9. TLS have been shown in both mouse and human kidneys10, and specific in the kidneys of patients with lupus nephritis (LN)11, 12, IgAN12, MGN12, 13, Pyelonephritis14, 15, Kidney clear cell carcinoma16, and rejected kidney grafts17, 18. We hypothesize that the development of TLS can be used in early detection of kidney diseases using in vivo imaging.
1.Aloisi, F. & Pujol-Borrell, R. Nat. Rev. Immunol 6, 205-217 (2006).
2.Neyt, K. et al. Trends Immunol 33, 297-305 (2012).
3.Pipi, E. et al. Front Immunol 9, 1952 (2018).
4.Dorraji, S.E. et al. Am J Pathol (2020).
5.Yin, C. et al. Front Immunol 7, 387 (2016).
6.Gonzalez Badillo, F.E. et al. Diabetes 68, 1990-2003 (2019).
7.Pikor, N.B. et al. J Immunol 198, 1775-1781 (2017).
8.Kendall, P.L. et al. J Immunol 178, 5643-5651 (2007).
9.Asam, S. et al. Rheumatology (Oxford) (2019).
10.Sato, Y. et al. Kidney Int 98, 448-463 (2020).
11.Chang, A. et al. J. Immunol 186, 1849-1860 (2011).
12.Luo, R. et al. Theranostics 11, 117-131 (2021).
13.Fleig, S.V. et al. Immun Inflamm Dis (2021).
14.Sato, Y. & Yanagita, M. Proc Jpn Acad Ser B Phys Biol Sci 95, 468-478 (2019).
15.Ichii, O. et al. J Am Soc Nephrol 33, 88-107 (2022).
16.Masuda, T. et al. J Immunother Cancer 10 (2022).
17.Dieude, M. et al. Am J Transplant 20, 726-738 (2020).
18.Nowocin, A.K. et al. Exp Clin Transplant 17, 330-338 (2019).