Lipid-based delivery systems for peptidomimetics
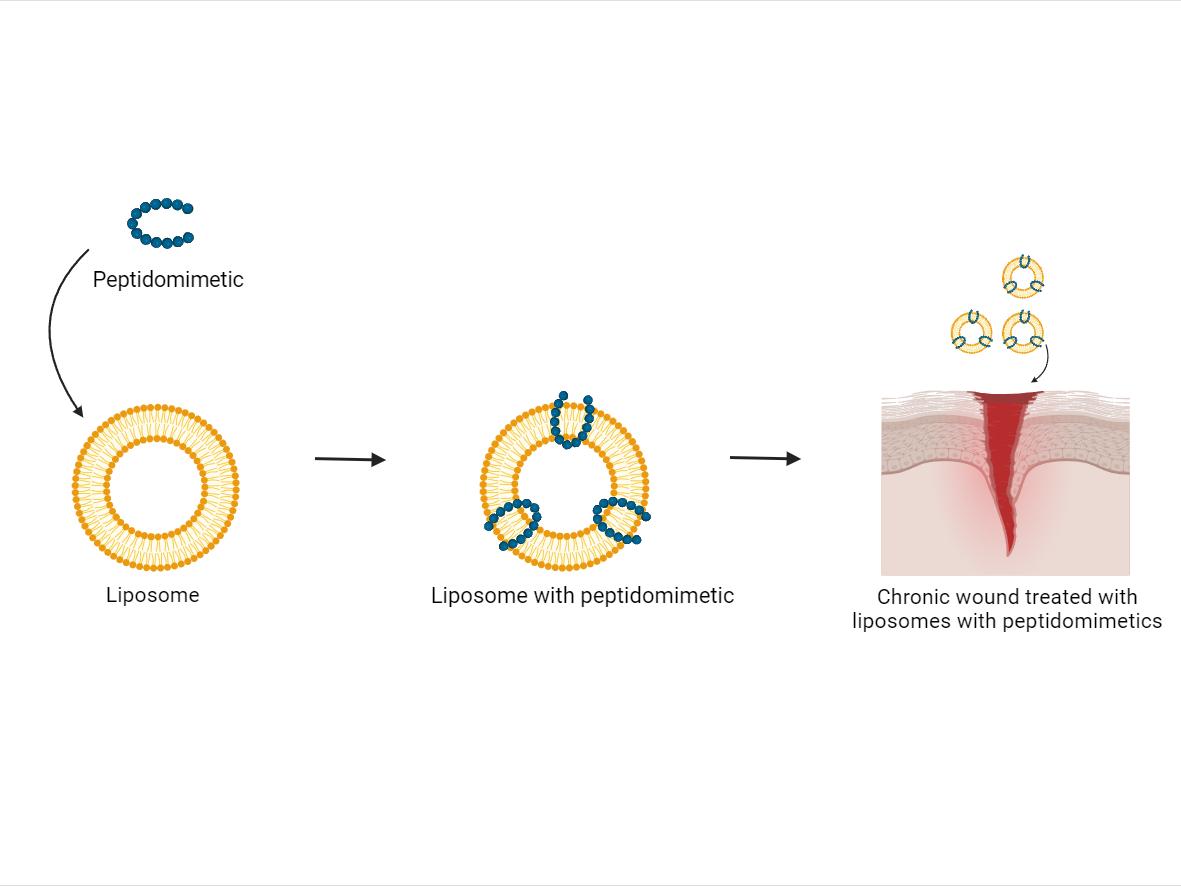 Figure 1: Illustration of peptidomimetic inserted into liposome creating a system of liposomes with peptidomimetics. These liposomes with peptidomimetics are then used to treat a chronic wound to improve the therapeutic outcome for patients. Figure created with BioRender.
Figure 1: Illustration of peptidomimetic inserted into liposome creating a system of liposomes with peptidomimetics. These liposomes with peptidomimetics are then used to treat a chronic wound to improve the therapeutic outcome for patients. Figure created with BioRender.Chronic wounds, characterized by a failure to progress through normal stages of healing within an expected timeframe, are significant healthcare challenges due to their high prevalence. Estimates suggest that approximately 1-2% of the general population will suffer from chronic wounds within their lifetime. Furthermore, numbers are steadily rising as the aging population grows and rates of diabetes, obesity, and vascular diseases increase. The consequences of infected chronic wounds pose a substantial burden on both individuals and healthcare systems; resulting in prolonged hospital stays, increased healthcare costs, and impaired quality of life for patients. Addressing the challenges of infected chronic wounds requires multidisciplinary efforts involving improved wound care practices, early detection strategies, and novel therapeutic strategies.
Antimicrobial peptidomimetics have emerged as a promising therapeutic approach for the treatment of infected chronic wounds. These compounds are designed molecules that mimic the structure and function of natural antimicrobial peptides, which play a crucial role in innate immunity and natural defence mechanisms. Furthermore, antimicrobial peptidomimetics have been found to exhibit immunomodulatory properties that can promote wound healing by accelerating angiogenesis and reducing inflammation. Ongoing research in this field aims to optimize the efficacy and safety profile of these novel therapeutics, thereby addressing the urgent need for new approaches in managing infected chronic wounds effectively.
The development of antimicrobial resistance has further complicated the management of chronic wounds. By encapsulating the peptidomimetics into liposomal structures, several advantages can be achieved. First, liposomes protect the peptidomimetics from enzymatic degradation and enhance their stability and bioavailability at the wound site. Moreover, liposomes can provide sustained release of antimicrobial agents, ensuring a prolonged therapeutic effect. Additionally, their small size could potentially enable enhanced penetration into bacterial membranes, boosting the efficacy against both planktonic and biofilm-associated bacteria commonly found in chronic wounds. Overall, this innovative therapeutic strategy holds great promise for effectively combating infections and promoting wound healing in patients with chronic wounds while minimizing systemic side effects often associated with traditional therapeutic regimes.
In our projects, we work with membrane-active compounds, such as peptidomimetics, to improve the current treatment strategies of chronic wounds. The aim is to develop lipid- and polymer-based drug delivery systems for localized treatment, especially in biofilm-infected wounds.