Structure elucidation and bioactivity screening and optimization of disulfide rich peptides
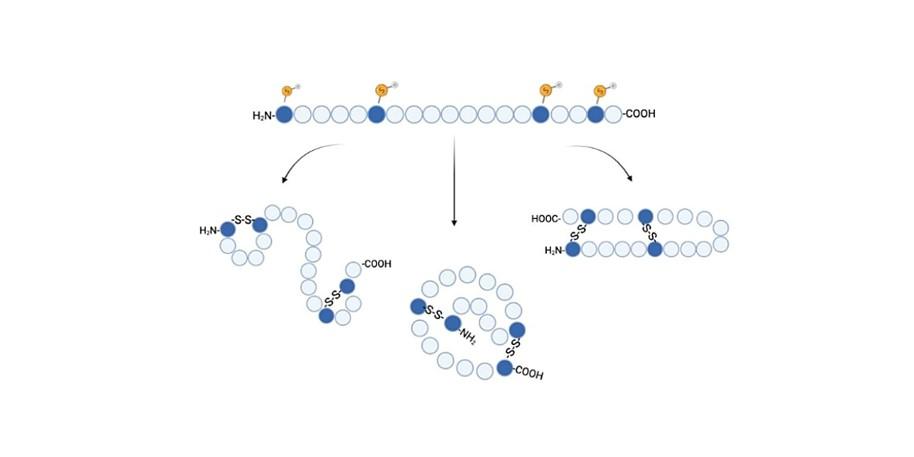 Figure 1: Illustration of how a peptide with two disulfide bridges can fold into three different 3-dimensional structures.
Figure 1: Illustration of how a peptide with two disulfide bridges can fold into three different 3-dimensional structures.The search for new bioactive peptides is essential for the development of new drugs to meet the antimicrobial drug resistance problems worldwide, and for the development of new and more efficient drugs. Bioactive peptides are specific protein fragments often found in nature via bioprospecting, which is the field of systematic search for chemical compounds in the biosphere. This project focus on Disulfide Rich Peptides (DRPs) that are a promising group of bioactive peptides and contain the amino acid cysteine, which forms disulfide bridges within the peptide. The disulfide bridges give the 3D structure of the peptide a higher chemical stability and also influence the bioactivity, and therefore determination of correct bridging in new peptides from bioprospecting is important if the peptides shall have a future as a potential drug or lead compound. The structural stability provided by the disulfide bridges also make DRPs better drug candidates than linear peptides that often display good activity in assays but lack the stability needed for e. g. oral administration.
The aim of the research project is to develop and improve methods for structure elucidation of bioactive peptides with antimicrobial and anticancer effect, and further to improve the bioactivity of the peptides by structure-activity relationship studies.