Blood Brain Barrier
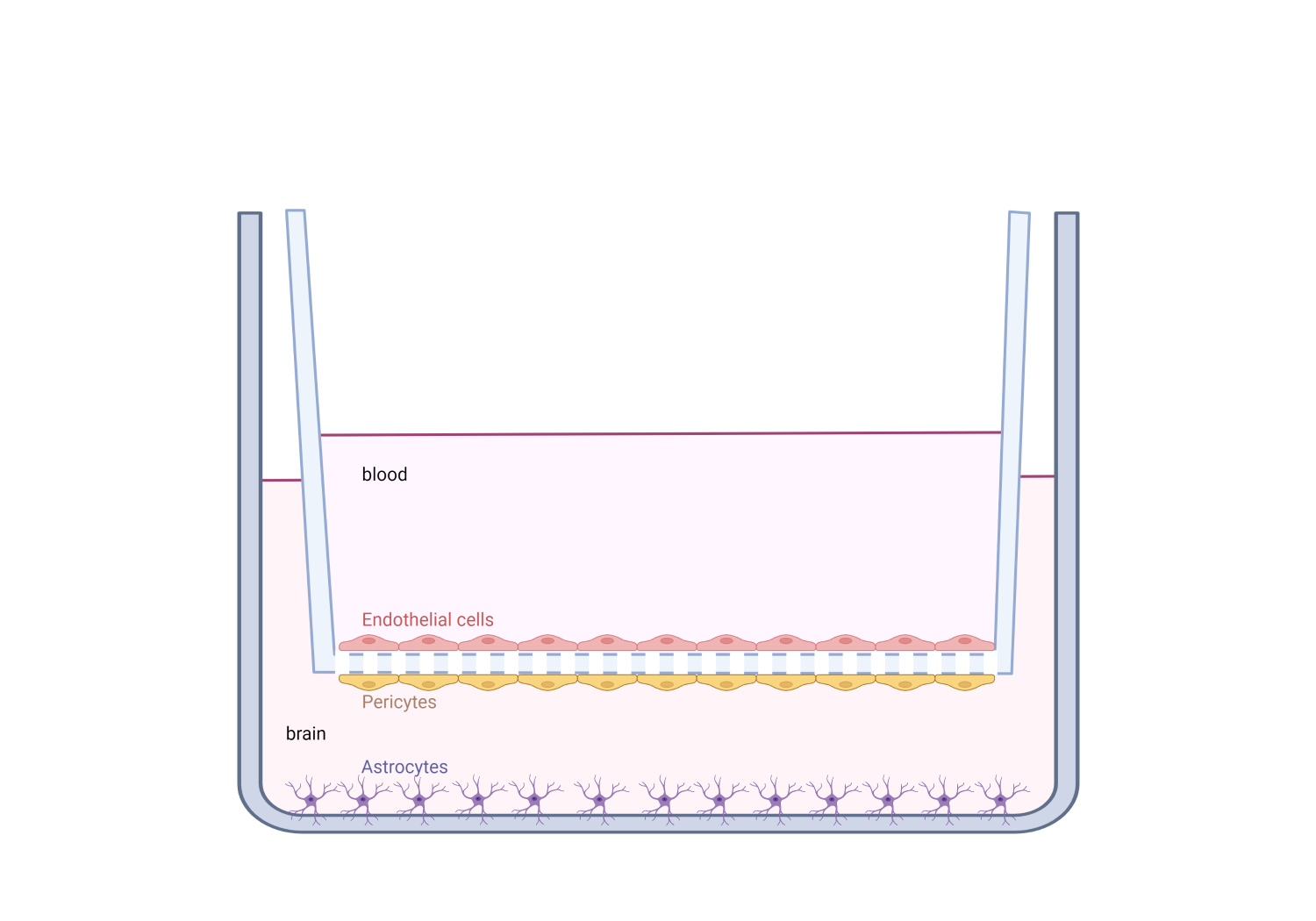
We believe that it may be possible to prevent FNAIT-associated ICH in the fetus by treating
pregnant women that have formed an immune response against the HPA-1a alloantigen, with
anti-HPA-1a monoclonal antibodies. The potential of anti-HPA-1a mAbs to protect the
integrity of the brain endothelium can be examined in an in vitro cell culture model of the
blood-brain-barrier (BBB)
PhD Candidate
Lennart Maximilian van Ligtenberg
2022-2026
PhD candidate at the Immunology Research Group. Here i work on fetal neonatal alloimmune thrombocytopenia, intracranial hemorrhage and oral tolerance as main research topics.
My teaching duties encompass laboratory courses, lectures, seminars, cases and supervision in courses like: MBI-2004, TPL-1010, SYD/SYP-1110, ERN-2009 & bachelor or master thesis subjects.
ICH is the most feared outcome of FNAIT and may result in stillbirth or neurological sequelae in the newborn. ICH occurs in around 10% of the FNAIT cases. For a long time, bleeding in FNAIT has been thought to be caused predominantly by platelet depletion. However, recent research has shown that anti-HPA-1a antibodies; have a direct effect on brain endothelial cells, could impair angiogenesis and contribute to cause ICH. The β3 chain is also expressed on endothelial cells in the αVβ3 heterodimer and can thus be bound by anti-HPA-1a antibodies. Remarkably, an anti-HPA-1a antibody subtype has been detected, which binds αVβ3 but not αIIbβ3 in FNAIT cases with ICH. This antibody was not found in cases without ICH. This study also showed that the αVβ3 specific anti-HPA-1a antibodies can induce apoptosis in endothelial cells, while β3 specific anti-HPA-1a antibodies could not induce apoptosis in endothelial cells. These findings indicate that ICH in FNAIT may be caused by αVβ3 specific anti-HPA-1a antibodies. From this view follows that the β3 specific anti-HPA-1a antibodies could be used a potential therapy for ICH as they can sterically hinder the binding of the αVβ3 specific anti-HPA-1a antibodies thus preventing ICH. Our lab has recently developed a human monoclonal anti-HPA-1a antibody that is of the β3 specific anti-HPA-1a antibodies (binds both αVβ3 and αIIbβ3) called mAb 26.4. mAb 26.4 was found not to inhibit angiogenesis in a tub forming assay and is thus a potential candidate for this type of treatment. In addition to the potential therapeutic effect of mAb 26.4 on endothelial cell in FNAIT associated ICH, mAb 26.4 was tested in a murine model and will soon be tested in clinical trials as a prophylactic treatment by induction of antibody mediated immune suppression (AMIS).
Members:
Financial/grant information:
Helse Nord & UiT