CARETOX
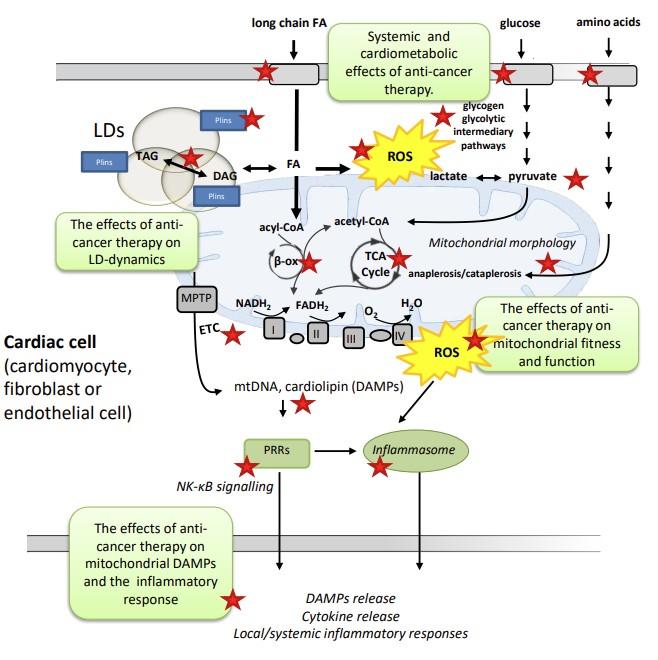 Illustration of work packages (WPs) associated with the CARETOX project. Red stars indicate steps and pathways that will be investigated in the project. Long chain fatty acids (FA) can enter the cell and either be stored as di-or triacylglycerol (DAG and TAG) or enter the β-oxidation to form acetyl-CoA which will enter the TCA-cycle. Overload of fatty acids in the cytosol may induce lipotoxicity and the formation of reactive oxygen species (ROS). Lipids can be stored in highly dynamic lipid droplets (LDs). Perilipins (Plins) are important regulators of lipid storage and play a role in reducing the lipotoxic burden on the heart. There is a crosstalk between LDs and mitochondria. Proper mitochondrial function and fitness is dependent on a mitochondrial electron transport system (ETS) containing respiratory complexes that form freely moving, diffusible entities assembled structures called “supercomplexes”. These complexes (I-V) provide both structural and functional advantages primarily through improved efficiency of oxidative phosphorylation and by the reduction of ROS emission. Mitochondrial stress may lead to the release of damage associated molecular patterns (DAMPs), such as mitochondrial DNA (mtDNA) and cardiolipin, which may activate pattern recognition receptors (PRRs). Activated PRRs will in turn initiate both local and systemic inflammatory responses.
Illustration of work packages (WPs) associated with the CARETOX project. Red stars indicate steps and pathways that will be investigated in the project. Long chain fatty acids (FA) can enter the cell and either be stored as di-or triacylglycerol (DAG and TAG) or enter the β-oxidation to form acetyl-CoA which will enter the TCA-cycle. Overload of fatty acids in the cytosol may induce lipotoxicity and the formation of reactive oxygen species (ROS). Lipids can be stored in highly dynamic lipid droplets (LDs). Perilipins (Plins) are important regulators of lipid storage and play a role in reducing the lipotoxic burden on the heart. There is a crosstalk between LDs and mitochondria. Proper mitochondrial function and fitness is dependent on a mitochondrial electron transport system (ETS) containing respiratory complexes that form freely moving, diffusible entities assembled structures called “supercomplexes”. These complexes (I-V) provide both structural and functional advantages primarily through improved efficiency of oxidative phosphorylation and by the reduction of ROS emission. Mitochondrial stress may lead to the release of damage associated molecular patterns (DAMPs), such as mitochondrial DNA (mtDNA) and cardiolipin, which may activate pattern recognition receptors (PRRs). Activated PRRs will in turn initiate both local and systemic inflammatory responses. Due to the profound advances in cancer therapy the last decades, the number of cancer survivors is increasing. Adverse cardiac side-effects such as heart failure, may however reduce the quality of life of many survivors and may even halter ongoing cancer therapy. Prevention strategies to reduce cancer therapy-induced cardiotoxicities have been attempted, but many have failed to demonstrate reliable effectiveness. Metabolic remodeling is well described in several forms of heart failure, often preceding the development of impaired cardiac function. The effects of cancer therapy on myocardial metabolism are however largely unknown. As cancer-cells are very different in terms of metabolism and substrate utilization compared to the highly oxidative beating heart cells, metabolic modulation could be a potential therapeutic target for reducing cardiac side-effects associated with cancer therapy.
In the CARETOX project we seek to elucidate how myocardial substrate utilization, lipid-dynamics, mitochondrial fitness, and cardiac inflammation are influenced by anti-cancer treatment.
An improved understanding of the underlying mechanisms can be used to provide better information to healthcare workers, patients and family, and may lead to the development of better prevention and treatment of such side-effects. Ultimately, the research in the CARETOX project has the potential to improve long-term cardiovascular outcomes and quality of life for breast cancer survivors and lead the way for new therapeutic approaches.
Members:
Financial/grant information:
AKM fond
Norwegian Health Association
UiT