The collaboration between the Department of Plastic and Reconstructive Surgery, the Department of Radiology and the Department of Gastroenterological Surgery has resulted in a number of innovative solutions for patients with chronic fistula where traditional techniques have failed.
News archive
14.07.2023:
Fistula closure by thinking outside the box
Fistula closure by thinking outside the box
The collaboration between the Department of Plastic and Reconstructive Surgery, the Department of Radiology and the Department of Gastroenterological Surgery has resulted in a number of innovative solutions for patients with chronic fistula where traditional techniques have failed.
Louis de Weerd 1,4
Sven Weum 2,4
Malgorzata Goschiewska 1
Stig Norderval 3
Jørn Kjæve 3
1 Department of Plastic and Reconstructive Surgery, University Hospital of North Norway
2 Department of Radiology, University Hospital of North Norway
3 Department of Gastroenterological Surgery, University Hospital of North Norway
4 Dermatoplastic Imaging Research Group, Department of Clinical Medicine, UiT the Arctic University of Norway
Corresponding author: Louis de Weerd (louis.deweerd@unn.no)
CLOSURE OF AN ENTEROCUTAN FISTULA WITH A MUSCLE FLAP
A fistula can be defined as an abnormal connection between two cavities covered by epithelium, in contrast to a sinus which is an abnormal passage that ends blindly. An enterocutaneous fistula is an abnormal connection between the intra-abdominal gastrointestinal tract (GI tract) and the skin. Enterocutaneous fistulas are associated with high morbidity and mortality, as well as significantly reduced quality of life for the patients. Malnutrition, electrolyte disturbances and septicaemia are important contributors to the high mortality. The prognosis has improved significantly after the introduction of total parenteral nutrition (TPN), which increases the incidence of spontaneous fistula closure and improves the nutritional status of patients requiring repeated operations.
Enterocutaneous fistulas occur most frequently as a complication of intra-abdominal surgery, but can also be caused by malignancy, inflammatory bowel disease, sequelae after radiation therapy or distal bowel obstruction. Acquired enterocutaneous fistula usually occurs postoperatively after accidental enterotomy or anastomotic leakage. Sitges-Serra et al. classified postoperative enterocutaneous fistulas into three main groups (1):
- Group 1: fistulas from the esophagus, stomach, and small intestine.
- Group 2: fistulas that drain via large defects in the abdominal wall.
- Group 3: fistulas from the appendix and colon.
These groups had a mortality rate of 8, 60 and 0% respectively. Fistulas in group 2 usually have a two-fold problem. Due to wound infection, wound rupture, intra-abdominal infections and repeated operations, there is often a large abdominal wall defect or abdominal wall without muscle in combination with a fistula. These will most often require surgical treatment and have a high mortality rate. Schein and Decker published their experience in a third-line care institution with 43 patients who had group 2 postoperative abdominal wall fistulas (2). They stated an overall mortality rate of 60% after surgical intervention. The volume of fluid produced by the fistula has been shown to be an independent predictor of mortality; high volume is defined as 500 mL per day. Our interdisciplinary collaboration for the treatment of enterocutaneous fistulas began with a patient in group 2, and resulted in an article in which we describe a technique we have chosen to call The Sandwich Design (3,4).
THE SANDWICH DESIGN
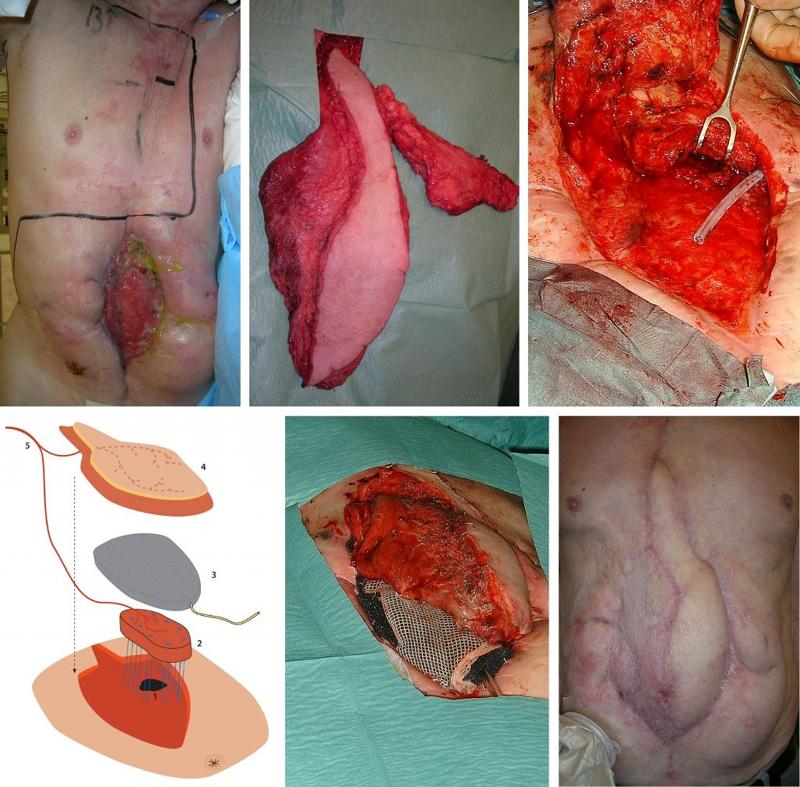
A 73-year-old man was admitted with severe abdominal pain and bleeding in the upper GI tract. The patient had previously been operated on several times in the ventricle, and had, among other things, undergone vagotomy and gastrojejunostomy. After endoscopy, a laparotomy was performed which showed cholecystitis with bleeding from the cystic artery and a fistula to the duodenum. The gastrojejunostomy was opened for inspection and then closed. Postoperatively, the patient developed a fistula from the gastrojejunostomy to the laparotomy wound. After several operations, including a ventriculostomy, at seven months the patient had a high-volume fistula from his gastrojejunostomy that emptied through an 11 cm x 4 cm abdominal wall defect (Figure 1a). There were no signs of distal intestinal obstruction on radiological examinations or at endoscopy. The fistula was located high against the costal arch. The upper abdominal wall up to the costal arch was covered with fibrosis due to leaks to the skin and repeated interventions. The patient was not able to undergo laparotomy and was placed on total parenteral nutrition.
A free flap consisting of both latissimus dorsi and serratus muscle was harvested on a pedicle from the thoracodorsal artery and vein. Only the lower three "fingers" of the serratus were included in the flap (Figure 1b). An anastomosis was made between the pedicle and the internal mammary vessels on the right side. The 3 cm x 5 cm fistula opening was closed with interrupted sutures. The serratus muscle flap was carefully fixed with sutures above this suture line, the pedicle was then placed upwards to protect it from possible leakage of aggressive intestinal fluid. The musculocutaneous latissimus dorsi flap, with a skin island of 10 cm x 5 cm, was placed over the serratus muscle and intestines to reconstruct the abdominal wall. The first three postoperative days passed without complications, but on the fourth day the fistula began to leak again. Massive leakage was observed every time the patient coughed.
At reoperation, the fistula of the gastrojejunostomy was completely opened (Figure 1c). The serratus muscle flap was inserted into the fistula opening and secured with interrupted sutures. A sponge for VAC (Vacuum Assisted Closure, KCI Medical, San Antonio, Texas, USA) was placed over the serratus and under the musculocutaneous latissimus dorsi flap (Figure 1d,e). The VAC system was set at 100 mm Hg continuous vacuum to immobilize the serratus to the fistula wall and counteract increased intra-abdominal pressure. A small part of the latissimus dorsi flap was covered with split skin graft. Total parenteral nutrition and nil per os were continued. Every two to three days the VAC sponge was changed under general anesthesia.
After two weeks, the sponge was gradually reduced in size. This allowed the latissimus dorsi to fuse to the serratus and the granulation tissue that covered the intestines. Endoscopy after three weeks showed complete coverage of the muscle that had been sewn into the fistula opening with mucosa from the ventricle/jejunum. The further postoperative course was complication-free (Figure 1f). Oral nutrition was introduced after five weeks, and the patient was discharged after eight weeks. There was no recurrence at follow-up after four years.
Interdisciplinary collaboration around our second patient with an enterocutaneous fistula, which could not be closed with standard procedures, resulted in a new technique we published under the name The Parachute Design (5).
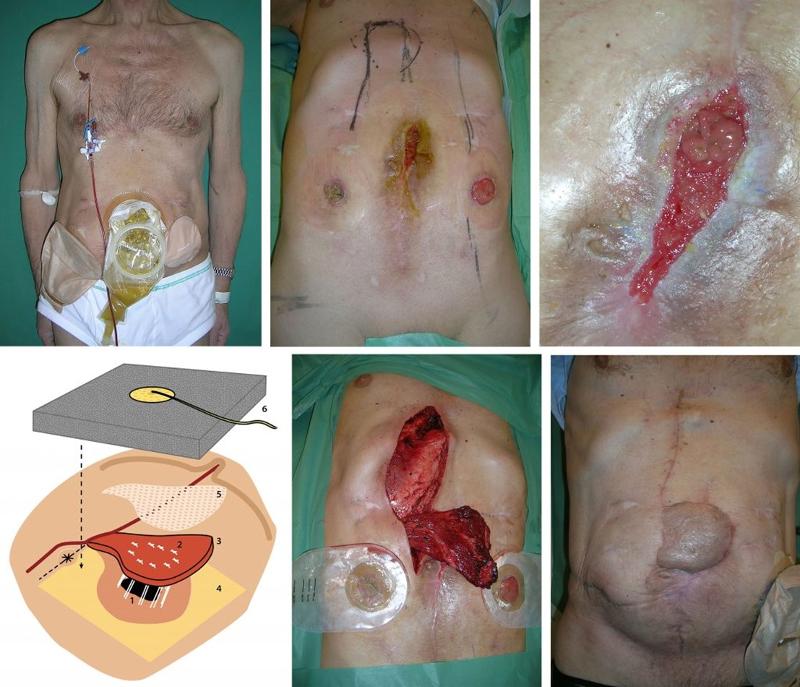
THE PARACHUTE DESIGN USING A PEDICLED RECTUS ABDOMINIS FLAP
A 67-year-old man had a complicated postoperative course after low anterior resection for rectal cancer. He developed an anastomotic leak and underwent six reoperations in nine months, including small bowel resection following accidental enterotomies. He finally received a sigmoidostomy and a cecostomy (Figure 2a). On examination, a high-volume jejunal enterocutaneous fistula with visible mucosa was found, draining through scar tissue in the midline (figure 2b).
The abdominal wall around the fistula was significantly damaged and consisted only of connective tissue. This fibrotic tissue had formed by secondary wound healing in a wound rupture after midline laparotomy. The patient was infection free and had a satisfactory nutritional status due to TPN. Distal bowel obstruction was ruled out as a cause of persistent fistula. Previous treatment with VAC had failed.
SURGICAL TECHNIQUE
The right musculus rectus abdominis was detached from the xiphoid process to 2 cm caudally of the umbilicus. The upper muscle attachment was detached, the superior epigastric vein and artery were ligated, and the muscle retained its vascular supply from the deep inferior epigastric artery. The muscle flap measured 20 cm x 7 cm. The skin around the fistula was de-epithelialized to a width of 3 cm. Furthermore, all scar tissue and other poorly circulated tissue in the fistula opening was routinely excised. This made the fistula opening larger, but this was subordinate to circulation in the area. The muscle flap was fixed with sutures into the fistula opening using the parachute technique. Nine resorbable Vicryl Plus 3-0 sutures (Johnson & Johnson, Piscatatway, NJ, USA) were inserted through the muscle and fistula wall into the intestinal lumen, through the fistula wall and muscle in a U-shape. The sutures were tied over the rectus abdominis (Figure 2c, d). The muscle was covered with a split skin graft. VAC (100 mm Hg) was used to immobilize the split skin graft and muscle flap to the fistula wall and abdominal wall. The patient was allowed to drink freely on the first postoperative day and eat on the second day. The VAC sponge was changed every five days for the first two weeks. The patient was discharged five weeks after the operation (Figure 2e). After two months, the cecostomy was closed. There was no recurrence at follow-up after twelve years.
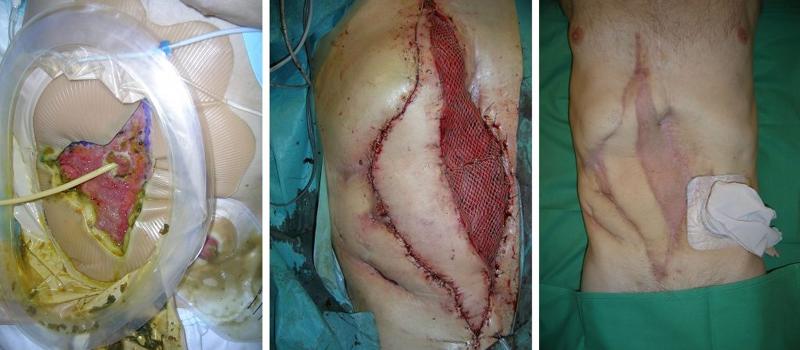
THE PARACHUTE DESIGN WITH FREE LATISSIMUS DORSI MYOCUTANEOUS FLAP
A 46-year-old man developed an enterocutaneous fistula after treatment for blunt abdominal trauma. In this case, it was not possible to use the rectus abdominis muscle flap. We therefore used a free latissimus dorsi myocutaneous flap to close the fistula and reconstruct the abdominal wall (Figure 3a, b, c).
High-risk patients with postoperative enterocutaneous fistula with little likelihood of spontaneous closure will benefit from close collaboration between plastic surgeon, gastroenterological surgeon, and radiologist. Final closure of the fistula can be achieved using The Parachute Design or The Sandwich Design, as this extraperitoneal approach allows tension-free closure of the fistula with a well-vascularized muscle flap, without compromising the bowel lumen. Early mobilization and initiation of enteral nutrition are further advantages of this technique (6).
We have used this extraperitoneal access in eleven patients. Before deciding to use one of these methods, it is important to rule out distal bowel obstruction, as this can prevent success, as we have seen in two of our patients. Radiological examinations are useful for accurately mapping the course of the fistula and ruling out distal bowel obstruction. Endoscopy may also be useful to rule out obstruction. We use CT angiography to map vascular anatomy and assess the quality of the rectus abdominis muscle, which may be damaged after previous surgery.
Since the technique is associated with little comorbidity, it would be advantageous to use it early in the course in patients with enterocutaneous fistulas who are not candidates for standard procedures. In the case of enterocutaneous fistulas, the aim is usually to remove the fistula-bearing segment of intestine via laparotomy. In addition, you must ensure that the drain is still open. It can be an extensive operation with loss of bowel and the possibility of post-operative complications.
Patients with difficult fistulas will usually need daily home care supervision, including enteral or parenteral nutrition. In the event of major leakage problems, this will also include regular admissions and calling home nursing care at all times of the day. The costs for home patients with fistulas can therefore be significant. At the same time, it is known that patients with major leakage problems from stomas or fistulas are often disabled with a very poor quality of life. If enterocutaneous fistulas can be closed without laparotomy, this should be considered, even if it requires quite extensive collaboration between different departments.
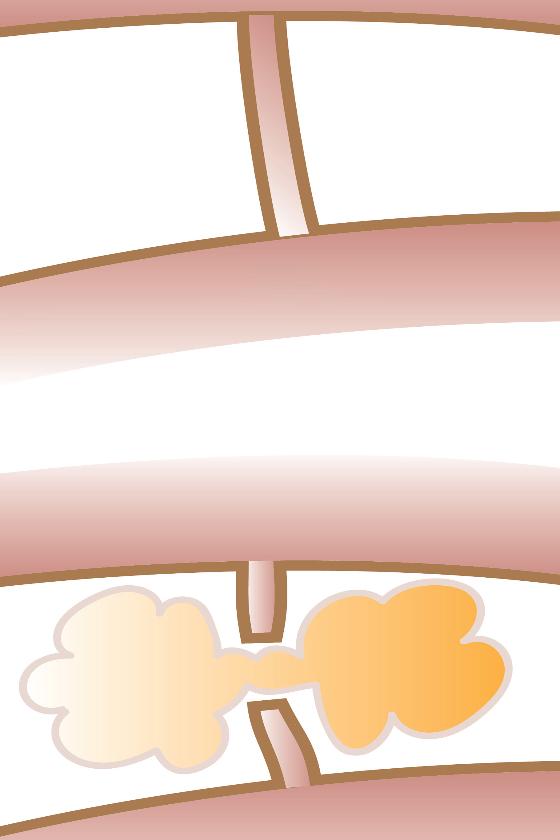
CLOSURE OF FISTULA WITH AUTOLOGOUS FAT TRANSPLANTATION
Perineogenital fistulas are not uncommon. In the Western world, they are usually due to postoperative complications, but such fistulas can also be caused by inflammatory bowel disease, infection, or sequelae after radiation therapy. In developing countries, they are usually due to complications after childbirth. The treatment is determined based on the cause and anatomical location. We use MRI preoperatively to map the course of the fistula and the local anatomy.
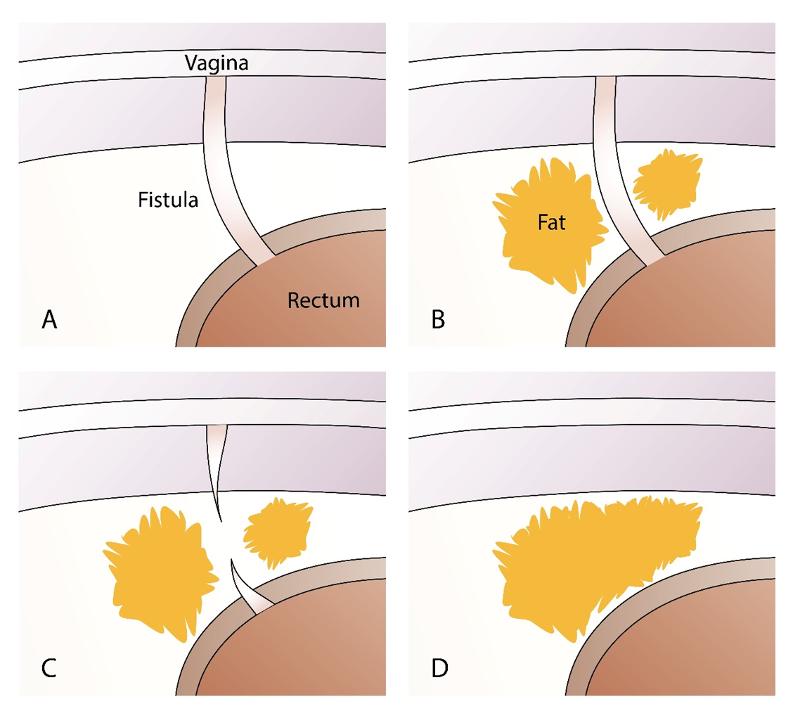
Fat transplantation is an established plastic surgery method for adding soft tissue. There is growing interest in the transplantation of fat within regenerative surgery since the method can also add stem cells. The fistulas often occur in areas where there is normally fat, but due to tissue damage the fat has been replaced by scar tissue. Our hypothesis was that autologous fat transplantation could become a new method for treating perineogenital fistulas (7,8,9). The mechanism of action should be to replace "like with like" and at the same time introduce stem cells to the area. When fat is transplanted, the scar tissue is infiltrated with fat tissue, at the same time as contractures caused by scar tissue are dissolved. A fistula is actually a contracture due to scar tissue, therefore it is important to dissolve the contracture when transplanting fat.
We transplant fat around the fistula after curettage of the fistula tract. The fat tissue that is transplanted exerts external pressure on the fistula tract, which is thus closed off (figure 4 ad). Curettage of the fistula tract causes raw wound surfaces, thus there is a permanent closure of the fistula tract when it heals. By cutting the fistula tract transversely, the contracture is resolved, while new fatty tissue is obtained between the two ends of the fistula tract. The fat provides mechanical support due to an increase in volume and can counteract high pressure from the intestinal side.
There are several reasons why injection of fat tissue can improve the healing of fistulas. In addition to adipocytes, the aspirate contains preadipocytes, mesenchymal stem cells, endothelial cells, fibroblasts and hematopoietic cells that have regenerative properties. This promotes neoangiogenesis, wound healing and tissue growth (10,11). In addition, mesenchymal stem cells will produce several substances with significant anti-inflammatory and immunomodulating properties in the area. Survival of transplanted fat is dependent on the number of cells that attach to the vascular network at the recipient site. The preadipocytes in the aspirate can divide and proliferate, they can differentiate spontaneously into adipocytes, and in this way contribute to the formation of new fat tissue.
The mechanisms behind the effects of fat transplantation are still partly unclear. Rehman et al. showed in an in vitro study that the secretion of vascular endothelial growth factor (VEGF) from human adipose stromal cells cultured under hypoxic conditions increased by a factor of five (7). Today, it is not known what stimulates this increase. We think that because of their tolerance to ischemia, it is especially the preadipocytes that survive after transplantation of fat to the areas of scar tissue around the fistula (11). After revascularization, the preadipocytes can proliferate and stimulate adipogenesis.
By using autologous fat transplantation when treating fistulas, the fistula tract itself is never excised, as this would create a large cavity. The transplant is done through two or three small incisions in the skin, each of which has a diameter of around 2 mm. Fat necrosis at the recipient site is a known complication. To reduce the risk of necrosis, it is important to distribute the fat evenly with many puncture channels at different levels, so that revascularization becomes possible. One must also avoid injecting too much fat, as increased pressure can cause local fat tissue necrosis.
The advantage of using autologous fat transplantation for fistula treatment is that the method is minimally invasive, and that one can still use other more established techniques if it is not successful. Cosmetically, this is also an optimal technique that adds soft, fresh tissue to the operated area.
AUTOLOGOUS FAT TRANSPLANTATION FOR RECTOVAGINAL AND ANOVAGINAL FISTULAS
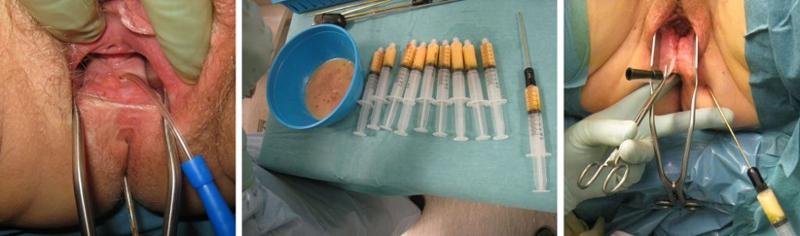
Our first patient was a young woman who developed a rectovaginal fistula after a complicated delivery. Several attempts at surgical closure had been made without success. Three years after the fistula debuted, the Department of Plastic Surgery was contacted. An adipofascial gluteal fold flap was used as an interposed graft. Although the fistula became significantly smaller in diameter, the patient still had significant fecal discharge from the vagina. After curettage of the fistula tract, a catheter was inserted into the fistula as a guide for autologous fat transplantation (Figure 5). After the fat was evenly distributed around the fistula, the fistula duct was transected with a sharp Coleman cannula for fat grafting before the catheter was removed. The vaginal side was closed with interrupted sutures. The postoperative course was uncomplicated. When checked after six weeks, exactly four years after the birth that caused the fistula, the patient had no passage of air or leakage of feces from the rectum to the vagina. Examination under general anesthesia showed that the fistula was healed. MRI showed that there was vital fatty tissue in the rectovaginal septum.
We published the successful results for our first six patients, and later the results of a two-center study with Aarhus University Hospital in Denmark (7,8). The technique had a success rate of 80% in a patient series that also included patients with Crohn's disease.
AUTOLOGOUS FAT TRANSPLANTATION IN RECTOURETHROPERINEAL FISTULA
A 68-year-old man developed a rectourethroperineal fistula after perineal prostatectomy where there was accidental damage to the rectum which was closed primarily with sutures. Conservative treatment did not lead to closure of the fistula. Neither excision of the fistula passage with layered closure, nor further surgery with a full-thickness sliding flap of the rectal wall resulted in healing. The patient was referred to the Department of Plastic Surgery to have an interponation of the gracilis muscle. With the same technique as described above, healing of the fistula was achieved after two autologous fat grafts six weeks apart. The technique is described in detail in a separate article (9).
AUTOLOGOUS FAT TRANSPLANTATION IN ANAL FISTULAS
A pilot study on autologous fat transplantation using the same technique as described above showed promising results. We have now completed a prospective two-center study in collaboration with Aarhus University Hospital. The results of the study are being processed for publication.
"The advantage of using autologous fat transplantation in fistula treatment is that the method is minimally invasive, and that you can still use other more established techniques if it is not successful."
A Norwegian version of this article was published in Kirurgen in 2020 and was granted the Best Article of the Year prize.
REFERENCES
1. Sitges-Serra J, Jaurrieta E, Sitges-Creus A. Management of postoperative enterocutaneous fistulas: the roles of parenteral nutrition and surgery. Br J Surg. 1982;69:147–150.
2. Schein M, Decker GAG. Gastrointestinal fistulas associated with large abdominal defects: experience with 43 patients. Br J Surg. 1990;77:97–100.
3. de Weerd L, Kjaeve J, Aghajani E, Elvenes OP. The sandwich design: a new method to close a high-output enterocutaneous fistula and associated abdominal wall defect. Ann Plastic Surg. 2007 May;58(5):580-3.
4. Gosciewska M, Jæve J, de Weerd L. Treatment of enterocutaneous fistulas with a free latissimus dorsi flap. Autumn meeting of the Norwegian Surgical Association, Session for free lectures Plastic surgery: 21-25 October 2019, Oslo, Norway.
5. de Weerd L, Kjæve J, Nergård S, The parachute design as a new method of closing a recalcitrant high-output enterocutaneous fistula; report of a case. Surg. Today. 2012 Jul;42(7):681-5.
6. Gosciewska M, Kjæve J, de Weerd L. Use of pedicled rectus abdominis and free latissimus dorsi flap in the treatment of enterocutaneous fistulas. The Autumn meeting of the Norwegian Surgical Association, Session for free lectures Gastrosurgery; 21-25 October 2019, Oslo, Norway.
7. de Weerd L, Weum S, Norderval S. Novel treatment of recalcitrant rectovaginal fistulas; barrel injection. Int Urogynecol J. 2015 Jan;26(1):139-44.
8. Norderval S, Lundby L, Hougaard H, Buntzen S, Weum S, de Weerd L Efficacy of autologous fat graft injection in the treatment of anovaginal fistulas. Tech Coloproctol. 2018 Jan;22(1):45-51.
9. de Weerd L, Norderval S, Weum S. Autologous fat grafting in the treatment of a rectourethroperineal fistula. Tech Coloproctol. 2013 Dec;17(6):677-8.
10.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298.
11. Von Heimburg D, Zachariah S, Low A, Pallua N (2001) Influence of different biodegradable carriers on the in vivo behavior of human adipose precursor cells. Plastic Reconstr Surg.
Lenke til nyhet
14.07.2023:
Ultrasound-guided injection of botulinum toxin for pain in the abdominal wall
Anterior cutaneous nerve entrapment syndrome (ACNES) is a major challenge causing disability, hospitalization and abdominal pain. In 2008 we developed an ultrasound-guided technique for precise injection of botulinum toxin at the point of nerve entrapment in the abdominal wall.
Ultrasound-guided injection of botulinum toxin for pain in the abdominal wall
Anterior cutaneous nerve entrapment syndrome (ACNES) is a major challenge causing disability, hospitalization and abdominal pain. In 2008 we developed an ultrasound-guided technique for precise injection of botulinum toxin at the point of nerve entrapment in the abdominal wall.
Sven Weum 1,2 and Louis de Weerd 1,3
1 Dermatoplastic Imaging Research Group, Department of Clinical Medicine, UiT the Arctic University of Norway.
2 Department of Radiology, University Hospital of North Norway.
3 Department of Plastic Surgery, University Hospital of North Norway.
Pain from the abdominal wall can be caused by nerve compression, a condition called anterior cutaneous nerve entrapment syndrome (ACNES). As alternatives to surgery, the condition can be treated with injection of local anesthetic, corticosteroids, or botulinum toxin. We have developed a new method for ultrasound-guided treatment of ACNES (1-3). With color Doppler, one can visualize the location where the perforator vessels and nerve exit through the anterior rectus fascia. Botulinum toxin can be injected below and above the anterior rectus fascia under ultrasound guidance. We have used the method as an alternative to surgery since 2008 without complications and it is now first-choice treatment for patients with ACNES at the University Hospital of North Norway (UNN). The method allows safe and accurate injection of botulinum toxin around the nerve where it is exposed to compression.
Background
Studies have shown that 10-30% of patients hospitalized with abdominal pain have pain from the abdominal wall without detectable intra-abdominal pathology (4). Chronic pain in the abdominal wall is often caused by ACNES (5). The surgeon John Berton Carnett (1890-1988) described a simple clinical test to distinguish between pain from the abdominal wall and pain with an intra-abdominal cause (4, 5). In the case of a positive Carnett's sign, the patient experiences increased pain when a pain point is palpated while the patient lifts the upper body from the examination table. Reduced or eliminated pain after injection of local anesthetic provides further support for the diagnosis of ACNES (4).
ACNES has traditionally been treated surgically with fasciotomy and neurectomy, or with injection of local anesthetic, corticosteroids or botulinum toxin into the pain points (5-7). Injection can be done blindly based on palpation findings, but imprecise placement of the needle can give uncertain results. Ultrasound-guided injection based on anatomical landmarks has previously been described (8), but this technique is only suitable for treating nerves that perforate the lateral part of the rectus muscle. It is our experience that ACNES is not limited to lateral nerve branches, many patients have pain centrally or medially over the rectus muscle. The pain in ACNES presumably occurs because nerves are compressed when they perforate the anterior rectus fascia (9).
From our experience with perforator flaps in reconstructive surgery, we know that the nerves to the abdominal wall follow both medial and lateral perforator vessels through the rectus muscle, which has also been shown in anatomical studies (10). With the help of color doppler, one can visualize the perforator vessels where they pass through the anterior rectus fascia, not only laterally but in all locations where perforators pass through the muscle. We have developed a method for treating ACNES with ultrasound-guided injection based on visualization of perforators.
Method
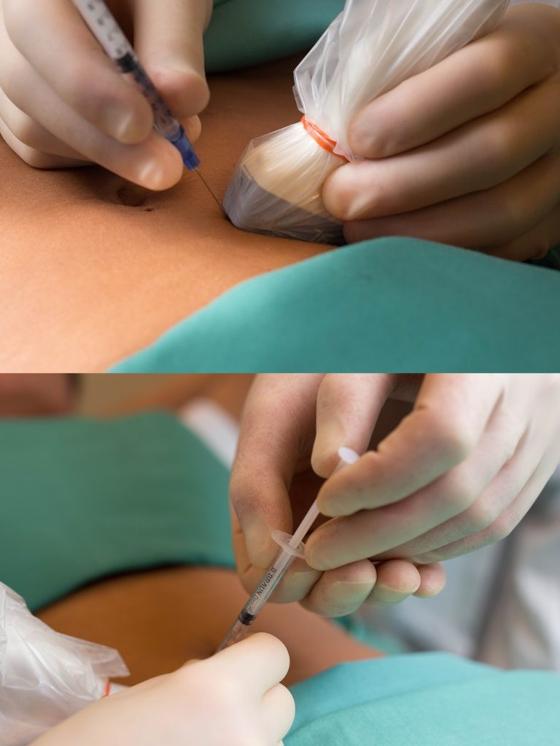
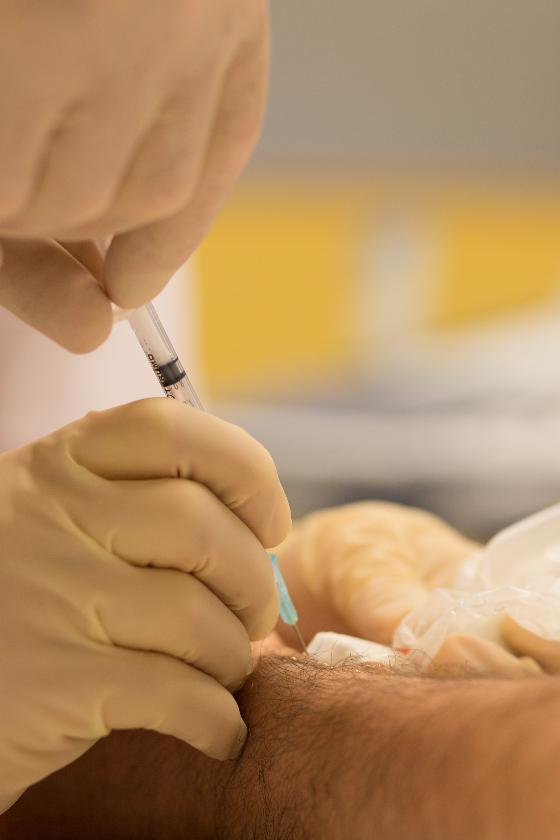
With the patient in the supine position, pain points in the abdominal wall are located with one finger and marked with a pen on the skin. After disinfection, the skin is examined with a linear ultrasound probe with a sterile cover. Color Doppler is used to visualize perforator vessels where these pass through the anterior rectus fascia (Figure 1).
It is important to be light-handed to avoid compression of the vessels. A 21 G needle is inserted through the skin at the short end of the probe and held in the image plane as it is advanced in the direction of the point where the perforator passes through the fascia. After aspiration to rule out an intravascular position, 1 ml of botulinum toxin 40 IU/ml is distributed below and above the anterior rectus fascia (Figure 2). The patient usually experiences increased pain as the needle tip approaches and when botulinum toxin is injected near the point where the nerve passes through the rectus fascia. The high concentration is used to avoid unnecessary spread of botulinum toxin. Therefore, we also do not add local anesthetic. This reduces the risk of botulinum toxin spreading away from the nerve and side effects such as muscle paralysis.
We published a study in Pain Medicine covering the period 2008-2014 when we treated 15 patients with 128 injections distributed over 46 consultations (1). Based on the results of the study, ultrasound-guided injection of botulinum toxin became first-choice treatment for ACNES at UNN. The average number of pain points per treatment was 2.7 and the average dose was 42 IU botulinum toxin per point. The maximum dose per consultation was 200 IU. In all patients, we could detect perforators that passed through the anterior rectus fascia in close relation to the pain points that were marked on the skin, these were located both medially, centrally, and laterally above the rectus muscle. Below the points where the patients had indicated pain, the perforator vessels were always visible on ultrasound with color Doppler, even if the nerves themselves could not be visualized. At follow-up, none of the patients had experienced side effects. Almost all patients stated that they had a good effect from the treatment and wanted a new injection when the effect of botulinum toxin decreased, usually after 2-4 months. One of the patients got rid of the pain completely after the first treatment and therefore did not need more injections.
Discussion
Injection of local anesthetic, corticosteroids or botulinum toxin are promising alternatives to surgery in ACNES (4-6). Since ACNES is related to compression where the nerve exits through the anterior rectus fascia, it is important that the cannula is placed precisely close to this point. Although it is possible to perform the injection blindly, it is not known whether the needle is in the correct position when injecting, which can have a significant impact on whether the treatment is effective. When injecting without the use of ultrasound, there is also no guarantee of avoiding an intra-abdominal injection. The fact that the pain increases when the needle tip approaches the point where the perforator passes through the rectus fascia provides indirect confirmation that the needle is in the same area as the nerve compression. Even if the patients have no prior knowledge of the location of the perforators in the abdominal wall, it turns out that the pain points they indicate correspond well with the location of the perforators where these penetrate the anterior rectus fascia.
Ultrasound-guided treatment based on anatomical landmarks has thus been described to find lateral nerves without the use of color Doppler. However, since the nerves are not visible on ultrasound, such a technique will not be able to treat ACNES which is caused by compression of nerves that penetrate the rectus muscle centrally or medially.
Plastic surgeons have made a significant contribution to the knowledge of the abdominal wall's perforators, since perforators are often used for blood supply in reconstructive surgery (11). The so-called perforator complex consists of an artery, one or two veins and a skin nerve (12). The method we have developed is based on our experience with sensate perforator flaps (13-15) and breast reconstruction with DIEP flaps (16). When dissecting perforator flaps, you can clearly see how the nerves follow the course of the blood vessels through or between the muscles (figure 3).
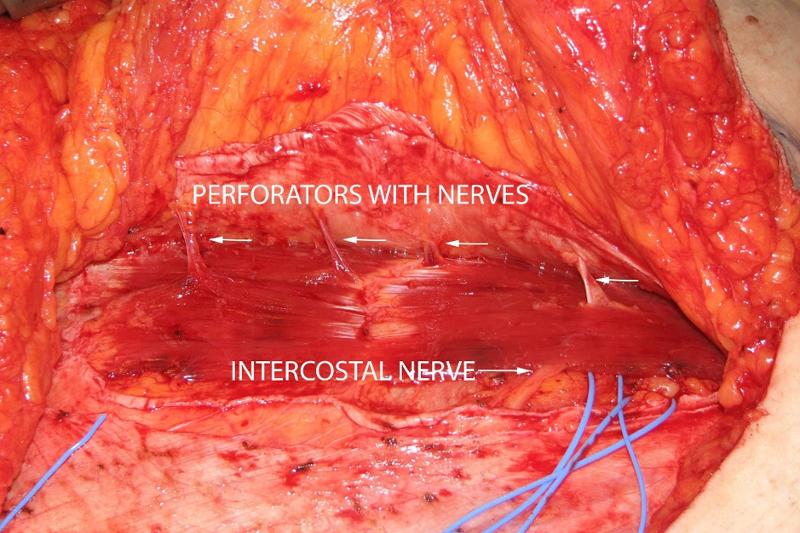
Our method for ultrasound-guided treatment of ACNES is based on this anatomical knowledge. Taylor et al. carried out an anatomical study which showed that the blood vessels are followed by nerves both medially and laterally in the rectus muscle (12). Yap et al. have made a dissection of perforators in the rectus muscle and have shown with histological examinations that nerve tissue is found together with the blood vessels in well over 90% of the perforators in preparations from both cadavers and clinical patients (10).
It is our experience that ACNES often involves compression of several skin nerves in the same patient. The patients do not only have pain along the lateral part of the rectus muscle, as several authors have claimed (7, 8, 17), many patients also have pain from nerves centrally and medially. Although ultrasound cannot visualize the nerves directly, color Doppler can clearly show the course of the blood vessels that join the nerves. The color Doppler technique makes it possible to inject botulinum toxin with great accuracy exactly where we think the nerve is compressed, namely where it exits through the anterior rectus fascia. We have now routinely used this technique at UNN since 2008 and have not registered any complications.
Conclusion
Ultrasound-guided treatment of ACNES with the use of color Doppler makes it possible to inject botulinum toxin with great accuracy around the nerve where it is subject to compression.
A Norwegian version of this article was published in Kirurgen in 2018.
References
- Weum S, de Weerd L. Perforator-Guided Drug Injection in the Treatment of Abdominal Wall Pain. Pain Med 2016; 17: 1229-32.
- Weum S, de Weerd L. Ultrasound of Small Nerves and Perforator-Guided Treatment of ACNES. Pain Med 2016; 17: 2439-40.
- Weum S, de Weerd L. Perforator-Guided Drug Injection at the Point of Nerve Entrapment. Pain Med 2017; 18: 1409-10.
- Lindsetmo RO, Stulberg J. Chronic abdominal wall pain–a diagnostic challenge for the surgeon. American journal of surgery 2009; 198: 129-34.
- Boelens OB, Scheltinga MR, Houterman S, et al. Management of anterior cutaneous nerve entrapment syndrome in a cohort of 139 patients. Annals of surgery 2011; 254: 1054-8.
- Jeynes LC, Gauci CA. Evidence for the use of botulinum toxin in the chronic pain setting–a review of the literature. Pain practice: the official journal of the World Institute of Pain 2008; 8: 269-76.
- Boelens OB, Scheltinga MR, Houterman S, et al. Randomized clinical trial of trigger point infiltration with lidocaine to diagnose anterior cutaneous nerve entrapment syndrome. The British journal of surgery 2013; 100: 217-21.
- Kanakarajan S, High K, Nagaraja R. Chronic abdominal wall pain and ultrasound-guided abdominal cutaneous nerve infiltration: a case series. Pain medicine 2011; 12: 382-6.
- Applegate WV, Buckwalter NR. Microanatomy of the structures contributing to abdominal cutaneous nerve entrapment syndrome. The Journal of the American Board of Family Practice / American Board of Family Practice 1997; 10: 329-32.
- Yap LH, Whiten SC, Forster A, et al. The anatomical and neurophysiological basis of the sensate free TRAM and DIEP flaps. Br J Plast Surg 2002; 55: 35-45.
- de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforator selection and planning of free DIEP flaps. Ann Plast Surg 2009; 63: 274-9.
- Taylor GI, Corlett RJ, Dhar SC, et al. The anatomical (angiosome) and clinical territories of cutaneous perforating arteries: development of the concept and designing safe flaps. Plastic and reconstructive surgery 2011; 127: 1447-59.
- de Weerd L, Weum S. The butterfly design: coverage of a large sacral defect with two pedicled lumbar artery perforator flaps. Br J Plast Surg 2002; 55: 251-3.
- de Weerd L, Weum S. The sensate medial dorsal intercostal artery perforator flap for closure of cervicothoracic midline defects after spinal surgery: an anatomic study and case reports. Ann Plast Surg 2009; 63: 418-21.
- de Weerd L, Solberg TK, Weum S. Closure of complex posterior midline defects after spinal surgery with sensate midline-based perforator flaps and the long-term results. Spine (Phila Pa 1976) 2015.
- Weum S, Mercer JB, de Weerd L. Evaluation of dynamic infrared thermography as an alternative to CT angiography for perforator mapping in breast reconstruction: a clinical study. BMC Med Imaging 2016; 4:43 p.m.
- Batistaki C, Saranteas T, Adoni A, et al. Ultrasound-guided anterior abdominal cutaneous nerve block for the management of bilateral abdominal cutaneous nerve entrapment syndrome (ACNES). Pain Physician 2013; 16: E799-801.
Lenke til nyhet
14.07.2023:
Lipedema - a new challenge for plastic surgeons
Lipedema almost exclusively affects women and cause significant discomfort and pain. When conservative treatment fails, water jet assisted liposuction with a vibrating cannula which preserves the lymphatic vessels is a promising surgical treatment. In 2018, we participated in a meeting with the Directorate of Health to investigate the possibilities of making lipedema treatment a public service in Norway. The report proposes that liposuction should be established as a trial treatment for a five-year period at a center in each health region.
Lipedema - a new challenge for plastic surgeons
Lipedema almost exclusively affects women and cause significant discomfort and pain. When conservative treatment fails, water jet assisted liposuction with a vibrating cannula which preserves the lymphatic vessels is a promising surgical treatment. In 2018, we participated in a meeting with the Directorate of Health to investigate the possibilities of making lipedema treatment a public service in Norway. The report proposes that liposuction should be established as a trial treatment for a five-year period at a center in each health region.
Louis de Weerd 1,3
Sven Weum 2,3
1 Department of Plastic and Reconstructive Surgery, University Hospital of North Norway
2 Department of Radiology, University Hospital of North Norway
3 Dermatoplastic Imaging Research Group, Department of Clinical Medicine, UiT the Arctic University of Norway
BACKGROUND
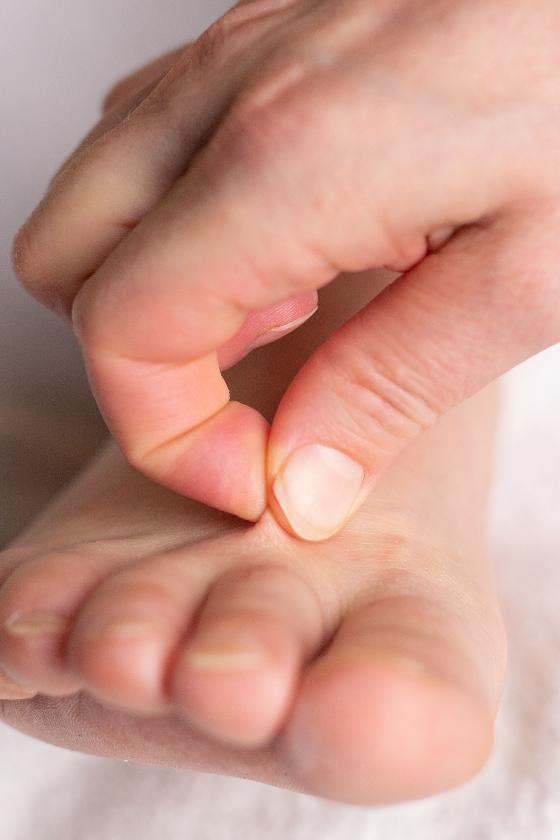
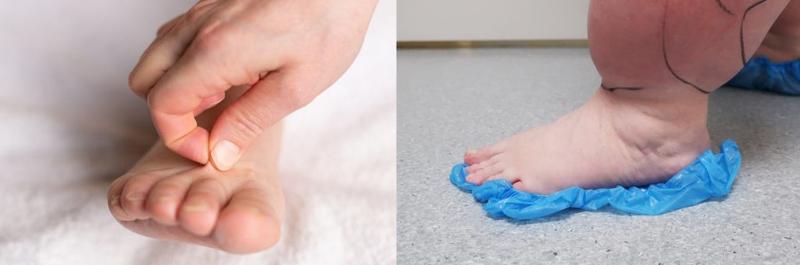
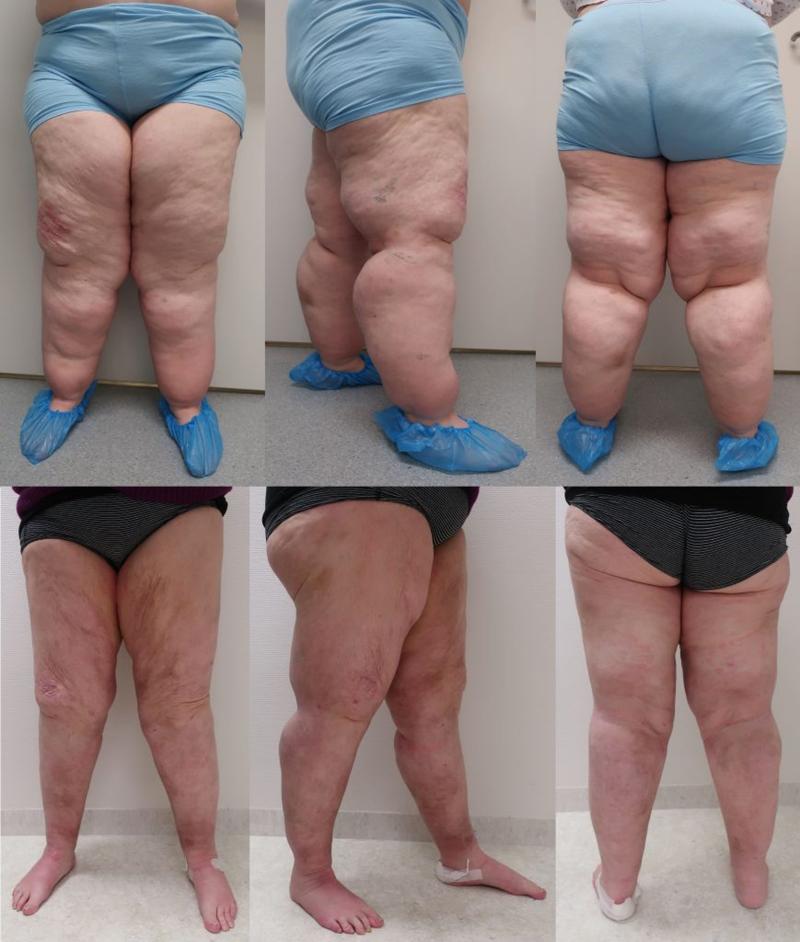
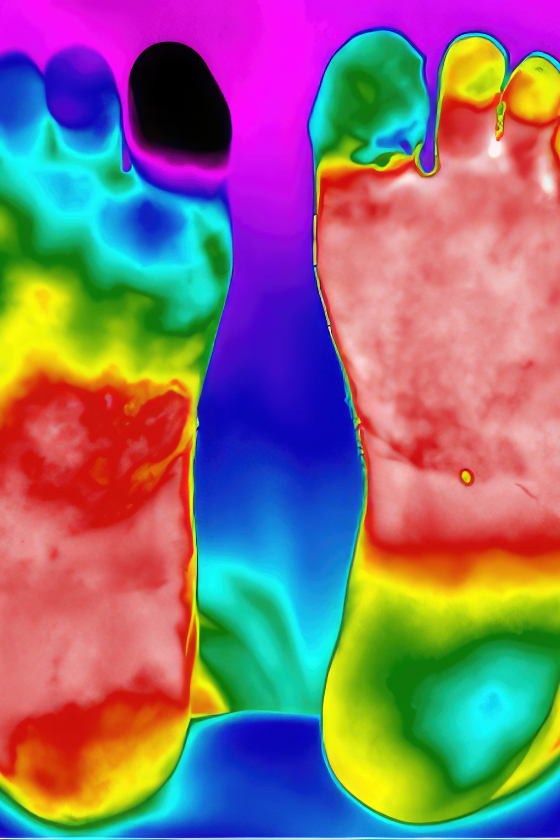
Lipedema is a chronic and usually progressive disease, which almost exclusively affects women and appears to have a hereditary component. The disease occurs insidiously, usually after puberty, and can cause significant discomfort (2-5). The first sign of lipedema is usually a disproportionate distribution of subcutaneous fat tissue in the lower limbs and seat area, slightly less frequently on the arms. Fat tissue increases in volume over time, but it is difficult to predict how quickly and by how much. Patients usually notice this towards the end of puberty, as there is an asymmetric distribution of fat between the upper and lower body, in contrast to lifestyle-related obesity which is usually evenly distributed between the upper and lower body. The affected areas are often tender or downright painful. The combination of disproportionate fat distribution and pain is completely characteristic of lipedema. However, gynecoid fat distribution or lipodystrophy are differential diagnoses with asymmetric fat distribution. Diet and physical activity have little effect on fat distribution. Lipedema always occurs bilaterally with symmetry between the right and left sides. The feet and hands are not affected, giving a typical cuff sign at the ankles and wrists (Figure 1). Unlike other forms of obesity, lipedema can cause severe pain and limit physical and social activities. The affected areas of lipedema can also be sensitive to touch and minor trauma. Patients bruise easily in these areas. Many describe a feeling of heaviness, tenderness or discomfort in the lower limbs. However, gynecoid fat distribution or lipodystrophy are differential diagnoses with asymmetric fat distribution. bilaterally with symmetry between the right and left sides. The feet and hands are not affected, giving a typical cuff sign at the ankles and wrists (Figure 1). Unlike other forms of obesity, lipedema can cause severe pain and limit physical and social activities. The affected areas of lipedema can also be sensitive to touch and minor trauma. Patients bruise easily in these areas. Many describe a feeling of heaviness, tenderness or discomfort in the lower limbs.
Most patients with lipedema have a high body mass index (BMI) and are either overweight or obese, while most have a normal appearance from the waist up. Obesity is an important risk factor with regard to the severity and prognosis of lipedema. Weight gain in lipedema is also affected by diet and physical exercise. Diet can influence obesity, but to a lesser extent on the pathological fat distribution. BMI can therefore be misleading in patients with lipedema since BMI only reflects total body mass and not the fat distribution itself. Despite high mean BMI, lipedema is associated with a low risk of diabetes, hyperlipidemia, and hypertension. Many patients experience that the skin is colder in the affected areas. The voluminous lower extremities are often misdiagnosed as lymphedema. Bed rest has only a limited effect on the lower limbs, indicating that the increased girth is mostly due to fat and not edema. However, large accumulations of fat in severe lipedema can also cause a secondary effect on the lymphatic circulation so that the patients get a so-called lipo-lymphedema, a serious and disabling condition. The ICD code for lipo-lymphedema is BD93.1 (lymphedema secondary to other specified cause).
EPIDEMIOLOGY
Little epidemiological research has been done on lipedema and there are no reliable data on prevalence in the population, but it is a rare condition. An unpublished epidemiological study from 2001 claims that lipedema occurs in 11% of women, but these data are based on patients referred to a clinic for lymphedema (6). There is therefore a strong possibility that this is an overestimate. However, this incidence has often been cited in the literature and stands in stark contrast to another study which describes the incidence as 1 in 72,000 in the population (7).
DIAGNOSIS
Correct diagnosis is essential to treat lipedema. Unfortunately, there are only a few clear criteria for the diagnosis of lipedema (2,3). The diagnosis is mainly based on clinical examination and medical history with family history. Disproportionality with a slim torso and thick lower limbs, as well as cuff signs on the ankles and wrists, are characteristic findings. Patients with lipedema often have pain in the affected areas, which are usually tender to the touch. On palpation, the transition between pathological and normal fatty tissue can be felt as a different texture. The affected fatty tissue often feels grainy, almost like sand, but the fat can also be nodular and feel like hard beans in a bean bag. Due to uneven fat distribution, some patients will develop gait disturbances,
Diagnosing lipedema in the early stages can be a big challenge. Sometimes absence of concavity on both sides of the Achilles tendon (filled lateral and medial malleolar groove) can be the only sign. Whether a swelling is caused by fluid or fatty tissue is primarily determined with the so-called pitting test. You press your thumb hard against the tissue for at least a minute; if there is a clear pit, there is fluid in the tissue. If there is only a modest impression, it is solid tissue / fatty tissue. There is usually no pitting edema unless the patient has also developed secondary lymphedema in late stages of lipedema. Positive Stemmer's test (not possible to pinch and pull up the skin over the dorsal side of the proximal phalanx of the second toe) helps to distinguish between lipedema and lymphoedema (Figure 1). Lipedema has been classified on the basis of skin changes and anatomical fat distribution. These classification systems do not say anything about how much trouble the patient has and thus only have value for describing the progression of lipedema over time (2). More information can be obtained by using the WHO's International Classification of Functioning, Disability and Health (ICF), which describes both symptoms and level of functioning (2,3). The WHO recommends this model because it is resource- and cost-effective when treating chronic conditions.
PATHOPHYSIOLOGY
There are no generally accepted evidence-based theories that describe the pathophysiology of the disease (2-4). The very term "lipedema" is also misleading, since it is not primarily edema (fluid accumulation in the tissue), but pathological distribution of fatty tissue. The fact that the disease runs in families indicates that genetics is important, possibly through X-linked dominant or autosomal dominant inheritance linked to gender (7). Since the condition primarily affects women and usually begins after puberty, possibly after pregnancy or at menopause, there is evidence for hormonal causes (3-5). It is still uncertain whether the pathological fat distribution is due to hyperplasia or hypertrophy of fat cells, or a combination of both. A thickened interstitium with increased interstitial fluid has been demonstrated in lipedema, which is most likely due to increased hydrostatic pressure. A clinical study from 2018 found increased sodium levels in the skin and subcutaneous tissue in women with lipedema (8). Activated adipogenesis in tissue with lipedema is believed to lead to hypoxia and thus necrosis of fat cells with subsequent recruitment of macrophages, a mechanism known in obesity (10,11). Histological examinations have shown microangiopathy which could be a possible cause of fragile capillaries and the leakage observed in lipedema patients who get bruises. a mechanism that is known in obesity (10,11). Histological examinations have shown microangiopathy which could be a possible cause of fragile capillaries and the leakage observed in lipedema patients who get bruises. a mechanism that is known in obesity (10,11). Histological examinations have shown microangiopathy which could be a possible cause of fragile capillaries and the leakage observed in lipedema patients who get bruises.
DIAGNOSTIC IMAGING
There are no specific imaging findings for lipedema (2,3). If lymphedema is suspected, the lymphatic drainage can be assessed with indocyanine green (ICG) fluorescence angiography or lymph scintigraphy. Although it has been common to use lymph scintigraphy to distinguish lipedema from lymphedema, a recently published study by Forner-Corder showed that there were changes on lymph scintigraphy in 47% of patients with lipedema. In other words, lipedema cannot be ruled out even if the examination shows changes in the lymphatic vessels (12). There is also no correspondence between diagnostic imaging and the patient's complaints. MR lymphangiography can produce anatomical and physiological changes in the lymphatic circulation (13). Both CT and MRI provide information on the amount and distribution of fat. Ultrasound can visualize the distribution of subcutaneous fat,
LABORATORY FINDINGS
There are currently no laboratory tests that can help diagnose lipoedema (3,4).
TREATMENT
There is little evidence for the treatment of lipedema. The treatment goals should be alleviation of the patient's symptoms as well as prevention of progressive disease and the development of lipo-lymphedema. Several authors recommend that the treatment should, as a minimum, consist of compression treatment, exercise and weight control (2-4).
CONSERVATIVE TREATMENT
Conservative treatment usually consists of compression treatment with stockings or bandages. A combination of physiotherapy and lymphatic drainage therapy is usually given to reduce the edema (complete decongestive therapy, CDT) if there is also edema (3,5,8). Although compression therapy has no effect on the amount of fatty tissue, many patients find that compression reduces tension and pain. Compression can also reduce orthostatic edema and improve walking function because a certain remodeling of the fatty tissue takes place (8). The patients thus become more mobile and function better in daily activities, which also has a positive effect in preventing obesity. It is important that the patients are referred to a physiotherapist who has experience with lipedema patients.
Exercise activates the muscle pump in the foot and leg, which in turn increases lymphatic drainage, reduces edema, and prevents obesity (3). Although no certain diet can prevent the pathological fat distribution, dietary changes are important for reducing symptoms and improving the prognosis. This is particularly important because obesity worsens the disease, so patients can benefit greatly from a nutritionist.
Women with lipedema often have psychosocial problems. The focus on body and weight combined with social shame linked to obesity predisposes to anxiety, depression, eating disorders and social isolation. It is therefore important that these patients receive follow-up on several levels, and many benefit from referral to a psychologist (2,3,4).
The wide range of ailments that affect lipedema patients means that the ICF classification is better suited for comprehensive mapping of the disease than lipedema-specific criteria alone. Lipedema is a chronic diagnosis, and The Chronic Care Model (CCM) can be useful in providing the best possible treatment. This model was originally introduced by Wagner in 1998 and has been shown to be useful in several other conditions, such as diabetes, neurological diseases, and atrial fibrillation (3,14). CCM identifies the essential ingredients of optimal treatment, this also includes self-treatment. Effective treatment requires that the patient's central role in the treatment is acknowledged, so that she gets a positive experience of autonomy and takes responsibility for her own health. The patient may benefit from being referred to a rehabilitation center.
SURGICAL TREATMENT
Unlike conservative treatment, liposuction can reduce the amount of fatty tissue. Before patients are offered liposuction, the obesity problem should be under control (3,5,8,15,16). Unfortunately, liposuction cannot cure the disease or remove the patient's predisposition to lipedema.
Liposuction is the least invasive method we have for removing fat from the affected areas. The technique is different from the techniques used in cosmetic liposuction. The patient should be informed that liposuction in her case is not a cosmetic procedure. It is important to use a gentle technique to preserve the lymphatic vessels during liposuction of lipedema patients (5,8,15,16). This includes the use of a vibrating cannula together with a liposuction machine or water jet assisted liposuction (WAL), use of special cannulas and longitudinal orientation of the puncture channels in the fat tissue. It has been described that such an approach not only reduces the volume of the lipedema, but that the disease progresses more slowly, and that the patients experience less pain, better walking function, increased mobility, and a higher quality of life (8,15,16). The importance of proper liposuction technique, including specific protocols for pre- and post-operative care, cannot be overstated for this patient group. This is particularly important because optimal treatment also reduces the risk of postoperative lymphedema. Furthermore, patients should also continue with a healthy diet and exercise, which is of great importance for general health and quality of life. The patients usually need compression treatment after the liposuction and lymphatic drainage if there is an indication for this (5,8,15,16). It should be a goal to achieve a normal weight after liposuction to prevent relapse after a few years. There is little data in the literature about the long-term results after liposuction for lipedema.
LIPEDEMA AND THE UNIVERSITY HOSPITAL OF NORTH NORWAY (UNN)
In 2016, we referred one patient with lipedema for treatment at a private clinic in Germany. The costs, which were covered by Helse Nord, totaled NOK 320,000. Later, referrals abroad have been coordinated centrally. Only treatment at public institutions that have an agreement with the Ministry of Health is now financially covered. When we referred the first patient to Germany, only private operators offered liposuction for the treatment of lipedema in Germany.
In 2016, we established an interdisciplinary outpatient clinic at UNN with a specialist physiotherapist and a plastic surgeon. After visiting Dr. Damstra at the publicly run specialist center for lymphoedema and lipedema in the Netherlands, we created a treatment plan focusing on self-management and conservative treatment with compression, weight control and physical exercise (Figure 2) (3). Patients who do not respond satisfactorily to this treatment, especially those who have a lot of pain, but who are physically active, have changed their lifestyle and achieved weight control, are offered water jet-assisted liposuction (Figure 3). This is usually performed as day surgery under sedation. Pre- and post-operatively, the patients receive intensive physiotherapy at UNN and with a local physiotherapist. All patients are followed up regularly.
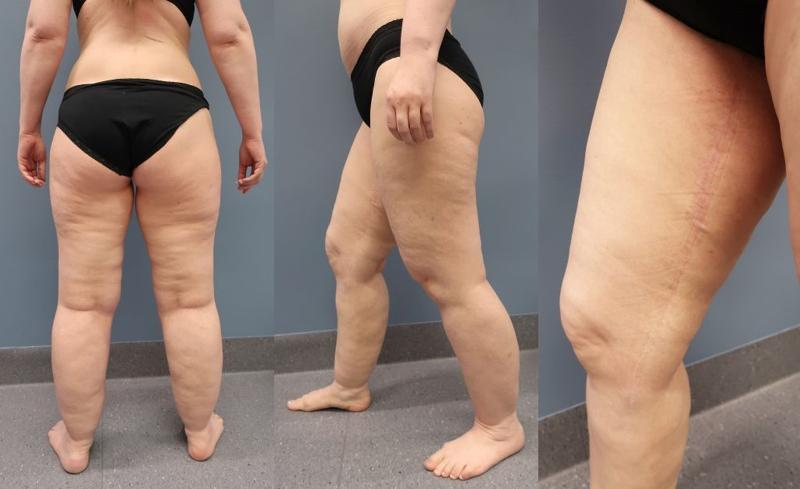
Since 2016, we have carried out 242 outpatient consultations (new referrals and controls), of which 135 in 2019. We have rejected 105 referrals, of which 74 were patients who did not belong to our health region (Helse Nord). As of today, we have 64 patients on the waiting list who will have an outpatient consultation before the summer. We have operated on 14 patients with an average of 2.5 operations per patient with water jet-assisted liposuction. There are 22 patients on the waiting list for surgery. Our preliminary results show that conservative treatment with a focus on self-treatment gives good results and has a positive effect on the quality of life. After water jet-assisted liposuction, patients report reduced pain and less tendency to bruise, greater physical activity and better quality of life. Lipedema is a new challenge in plastic surgery. Our experiences at UNN with the use of water jet-assisted liposuction show, in the same way as the published studies, that the treatment has a significant effect on the quality of life. All patients who were on sick leave due to lipedema and underwent liposuction returned to work after the treatment. It is therefore important to establish a public service for this patient group.
NATIONAL SERVICE?
In 2018, we participated in a meeting with the Directorate of Health to investigate the possibilities of making lipedema treatment a public service in Norway. In 2019, the health authorities were commissioned to investigate whether surgical treatment should be established as a public service for this patient group (17). The investigation was carried out by the plastic surgery community in collaboration with physiotherapists who have experience with lipedema, and the report was handed over to the authorities in March 2020. The report proposes that liposuction should be established as a trial treatment for a five-year period at a center in each health region. There must be clear joint inclusion criteria for treatment and an accompanying joint quality register. The treatment must be evaluated at the end of the period.
The Ministry of Health and Care Services is positive about the proposals and recommends that treatment should preferably be offered to patients with lipedema, preferably through a clinical study. How this is to be organized is left to the regional health organizations.
A Norwegian version of this article was published in Kirurgen in 2020.
REFERENCES
1. Allen EV, Hines EA Jr. Vascular Clinics X. Lipedema of the legs: A syndrome characterized by fat legs and orthostatic edema. Proc Staff Mayo Clinic 1940;15:184-187
2. Bertsch T, Erbacher G, Corda D, Damstra RJ, et al. Lipoedema.myths and facts, Parts 5. Phlebologie 2020;49;31-50
3. Halk AB, Damstra RJ. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. 2017;32:152-1
4. Reich-Schpke S, Alrmeyer P, Stücker M. Thick legs-not always lipedema. JDDG;2013;103:225-232
5. Buck DW 2nd, Herbst KL.Lipedema: A relatively common disease with common misconceptions. Plast Reconstr Surg Glob Open 2016 Sep 28;4(9):e1043. eCollection 2016 Sep
6. Földi M, Földi E. Földi's Textbook of Lymphology. 2nd ed. San Francisco: Elsevier, 2006
7. Child AH, Gordon KD, Sharpe P, et al. Lipedema: an inherited condition. Am J Med Genet A; 2010;152:970-976.7
8. Stutz J. Liposuction beim Lipödem zur Verhinderung von Gelenkspätkomplikationen Vasomed 2011 Volume 23 (ISSN: 0942-1181)
9. Crescenzi R, Marton A, Donahue PMC, et al. Tissue Sodium content is elevated in the skin and subcutaneous adipose tissue in women with lipedema. Obesity (Silver Spring) 2018;26:310-317
10. Bauer AT, von Lukowicz D, Lossagk K, et al. New insight on lipedema; The enigmatic disease of the peripheral fat.. Plast Reconstr Surg. 2019 Dec;144(6):1475-1484
11. Bauer AT, von Lukowicz D, Lossagk K, et al. Adipose stem cells from lipedema and control adipose tissue respond differently to adipogenic stimulation in vitro. Plastic Reconstr Surg. 2019 Sep;144(3):623-632
12 Forner-Cordero I, Oliván.Sasot P, Ruiz-Llorca C, Munoz-langa J. Lymphoscintigraphic findings in patients with lipedema. Rev Esp Med Nucl Imagen Mol. 2018;37:141-148
13. Lohrmann C, Foeldi E, Langer M. MR imaging of the lymphatic system in patients with lipedema and lipo-lymphedema. Microvasc Research 2009;77:335-339
14. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract 1998;1:2-4)
15. Sanhofer M, Hanke CW, Habbema L, Podda M Rapprich S, Smeller W. Prevention of progression of lipedema with liposuction using tumescent local anaesthesia: Results of an international consensus conference. Dermatol Surg 2020;46:202-228
16 Peprah K, MacDougall D. Liposuction for the treatment of lipedema: A review of clinical effectiveness and guidelines. Ottawa: CADTH; 2019 Jun. (CADTH rapid response report: summary with critical appraisal) ISSN:1922-8147(online)
17. Should a public surgical treatment service be established for patients with lipedema? (Bør det etableres et offentlig kirurgisk behandlingstilbud til pasienter med lipødem?) Public report to the Ministry of Health and Care, delivered March 12th, 2020.
Lenke til nyhet

06.07.2023:
Evaluation of microcirculation in flap surgery with thermography
At the University Hospital of North Norway (UNN), thermography has been used in the Department of Plastic and Reconstructive Surgery for almost two decades, and the Department of Radiology has established a separate laboratory for medical thermography. The thermal camera can be used to locate perforators under the skin, e.g. in connection with breast reconstruction.
Evaluation of microcirculation in flap surgery with thermography
At the University Hospital of North Norway (UNN), thermography has been used in the Department of Plastic and Reconstructive Surgery for almost two decades, and the Department of Radiology has established a separate laboratory for medical thermography. The thermal camera can be used to locate perforators under the skin, e.g. in connection with breast reconstruction.
Louis de Weerd 1,3
Sven Weum 2,3
James B. Mercer 2,3
1 Department of Plastic and Reconstructive Surgery, University Hospital of North Norway
2 Department of Radiology, University Hospital of North Norway
3 Dermatoplastic Imaging Research Group, Department of Clinical Medicine, UiT the Arctic University of Norway
Humans maintain a constant body temperature despite changing temperatures in their surroundings. This is possible because there is a fine balance between heat production and heat loss. Heat production occurs through metabolism and muscle contraction. The heat produced is transported further through the body via the blood circulation. The body's temperature regulation, which keeps the core temperature relatively constant, is a complicated process, and the skin plays an important role in this regulation. More than 30% of the body's temperature-sensitive receptors are found in the skin. Heat loss to the surroundings occurs through conduction, convection, evaporation and heat radiation (figure 1) [1].
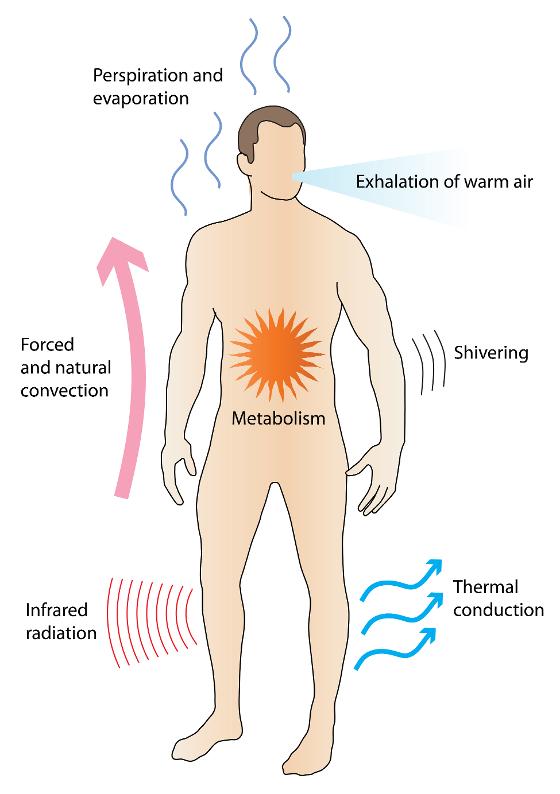
Under stable ambient temperatures between 18 and 25 degrees Celsius, the most important mechanism of these is heat radiation from the skin to the surroundings. Such heat radiation occurs in the form of infrared radiation [2,3] which is part of the electromagnetic spectrum and has a wavelength from 1 millimeter to 750 nanometers, i.e. the area between microwaves and visible light. Although infrared radiation is invisible to the eye, the radiation is felt as heat.
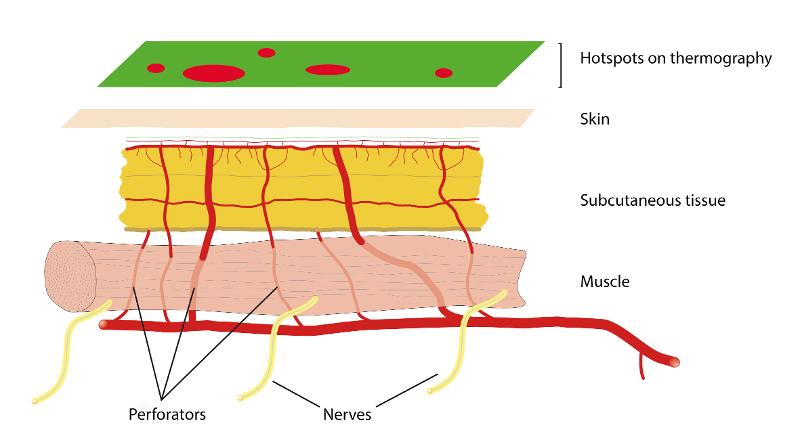
Medical infrared thermography
Skin temperature has long been used as an indicator of possible pathology. There is a defined relationship between the skin temperature and infrared radiation from the skin's surface. Studies have also shown that in humans there is a good correlation between the temperature of the skin and the degree of perfusion [4,5,6]. Measurement of skin temperature can therefore give us useful information about the skin's perfusion.
The infrared radiation from the skin surface can be recorded with a thermal camera. In medical infrared thermography, such a camera is used to create images where different colors represent different temperatures on the skin surface. Modern thermal cameras are very accurate and can measure the skin's temperature and visualize temperature differences of less than 0.1 degrees Celsius. With today's digital thermal cameras, the images can be analyzed and processed on an ordinary laptop computer. Medical infrared thermography is currently used in many specialties such as neurology, rheumatology, oncology, cardiovascular surgery, orthopedics and plastic surgery [1,3].
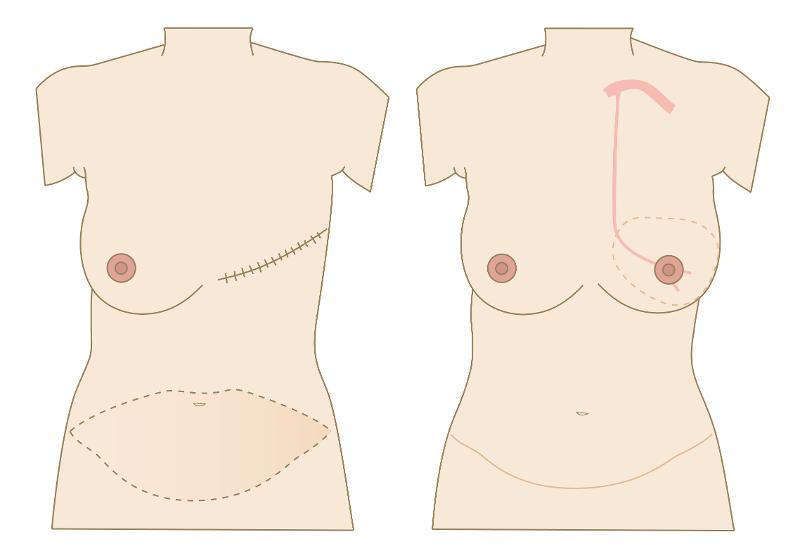
Breast reconstruction with a free DIEP flap
In breast reconstruction with a free DIEP flap, skin and fatty tissue from the abdomen are used to reconstruct a new breast (figure 3). The new breast receives its blood supply via a so-called perforator (figure 4). This consists of a small artery and one or two veins that originate from the deep inferior epigastric artery and vein. When the flap is moved to the chest wall, these vessels are connected to the internal mammary artery and vein. For these interventions, we currently use thermography pre-, peri- and postoperatively.

Preoperative DIRT
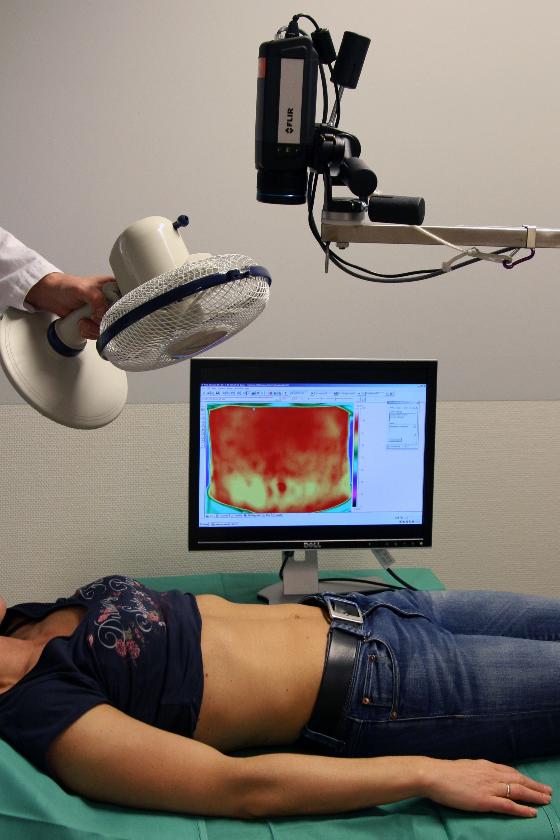
When planning a DIEP flap, it is important to find a good perforator that will supply the flap after breast reconstruction. Since 2006, it has become very common to use CT angiography to get an overview of the various perforators in the abdominal wall. Disadvantages of CT are the high dose of X-rays and the need to use intravenous contrast. In the last ten years, we at UNN have therefore used DIRT when planning such operations. The technique was described as early as 1993 by Itoh and Arai [7]. After cooling down, clear hot spots are seen on the skin where circulation returns. Our research has shown that the hotspots that emerge fastest and heat up the largest areas of skin are above perforators that are also suitable for supplying a DIEP flap. The hotspots correspond well with the location of perforators on CT, arterial sound with handheld Doppler and intraoperative findings [8,9]. Figure 5 shows the use of the technique during the planning of a DIEP reconstruction.

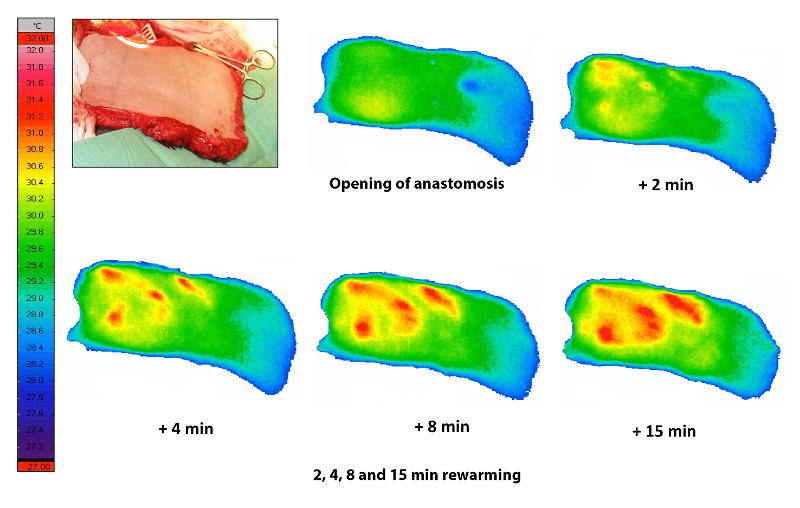
Intraoperative DIRT
When the DIEP flap is harvested, it is very easy to damage the perforators [10]. Therefore, it is very important to know if a perforator is working adequately. After the flap has been harvested, but still receives its blood supply from the abdomen, we can use DIRT to test whether the flap has been circulated through the perforator. To cool down the skin, we simply use a sterile metal plate at room temperature. The thermal camera then records if adequate heating takes place afterwards. When the flap has been moved up on the chest wall and the blood vessels have been anastomosed, it is important to see that blood circulation is restored. Now the flap is already cooled down since it has been without circulation for a while. With the thermal camera, we can quickly see if the circulation returns. In the case of a inadequate circulation, it is important to recognize this as quickly as possible, preferably during the operation and not later. The flap has a heating pattern with hot spots (figure 6). We have shown that with venous stasis this pattern will disappear, and the flap will be diffusely heated [11]. This is how we can distinguish between arterial and venous circulatory failure. In some cases, it is necessary to connect an additional vein to reverse the venous stasis. At the end of the operation, we use DIRT to confirm that there is circulation in the flap.

Postoperative DIRT
In the postoperative phase, it is very important to monitor that the flap has an adequate blood supply. The usefulness of measuring the flap's temperature has been debated. When you look at thermography images, it is necessary to measure the temperature at exactly the same point each time, because there are areas with hotspots and other areas that have a lower temperature. We have used thermography to look at the perfusion dynamics in DIEP flaps in the first week after surgery [12]. It turns out that the perfusion in a DIEP patch is a dynamic process. In the first few days, there is a gradual increase in perfusion in the flap, this eventually stabilizes. In some cases, especially when the skin is very pale, one may doubt whether the arterial anastomosis is working. Then DIRT can quickly give a reliable answer to this (figure 7).
Conclusion
The microcirculation in DIEP flaps can be easily monitored with DIRT. The technique is easy to use, it does not produce ionizing radiation and does not require the use of intravenous injection. Preoperatively, DIRT can be a good alternative to CT. Peri- and postoperatively, the method provides valuable information about the flap's perfusion.
A Norwegian version of this article was published in Kirurgen in 2012.
References
- Jiang LJ, Ng EYK, Yeo ACB, et al. A perspective on medical infrared imaging J Med Eng Tech 2005;29:257-267.
- Head JF, Elliott RL. Infrared imaging; making progress in fulfilling its promise. IEEE Eng Med Biol Mag 2007;21:80-85.
- Ring EF, Ammer K. Infrared thermal imaging in medicine. Physiol Meas. 2012;33:R33-46.
- Awwad AM, White RJ, Webster MHC, et al. The effect of temperature on blood flow in island and free skin flaps: an experimental study. Br J Plast Surg 1983;36:373-82.
- Mercer JB, de Weerd L. The effect of water-filtered infrared-A (wIRA) irradiation on skin temperature and skin blood flow as evaluated by infrared thermography and scanning laser Doppler imaging. Thermol Int 2005;15:89-94.
- Merla A, Di Romualdo S, Di Donato L, et al. Combined thermal and laser Doppler imaging in the assessment of cutaneous tissue perfusion. Conf Proc IEEE Med Biol Soc S 2007; 2530-2533.
- Itoh Y, Arai K. The deep inferior epigastric artery free skin flap : anatomical and clinical application. Plastic Reconstr Surg 1993;91:853-863.
- de Weerd L, Weum S, Mercer JB. The value of dynamic infrared thermography (DIRT) in perforator selection and planning of DIEP flaps. Ann Plast Surg 2009;63:274-279
- Whitaker IS, Lie KH, Rozen WM, et al. Dynamic infared thermography for the preoperative planning of microsurgical breast reconstruction: A comparison with CTA. J Plast Reconstr Aesth Surg 2012;65:130-132
- Busic V, Das-Gupta R, Mesic H, Begic A. The deep inferior epigastric perforator flap for breast reconstruction, the learning curve explored. J Plast Reconstr Aesthet Surg 2006;59:580-584.
- de Weerd L, Mercer JB, Bøe SL. Intraoperative dynamic infrared thermography and free-flap surgery. Ann Plast Surg 2006;57:279-284
- de Weerd L, Miland ÅO, Mercer JB. Perfusion dynamics of free DIEP and SIEA flaps during the first postoperative week monitored with dynamic infrared thermography (DIRT). Ann Plast Surg 2009;62:40-47.
Lenke til nyhet
29.06.2023:
Dynamic infrared thermography
Before the Dermatoplastic Imaging Research Group (DIRG) was established, there had been close collaboration for several years between the departments of radiology and plastic surgery in the development of dynamic infrared thermography (DIRT) as a new imaging technique.
Dynamic infrared thermography
Before the Dermatoplastic Imaging Research Group (DIRG) was established, there had been close collaboration for several years between the departments of radiology and plastic surgery in the development of dynamic infrared thermography (DIRT) as a new imaging technique.

For more than a decade Professor James Mercer has been responsible for the thermography laboratory, recently in a position as Professor Emeritus. DIRT is a unique modality not offered by any other European departments of radiology. The scientific research on DIRT in Tromsø has produced several PhD- and Master degrees.
The research group has a leading role on the use of DIRT in reconstructive surgery, the group has provided at scientific basis for the clinical use of DIRT in the planning of surgery as well as intra- and postoperatively in perforator flap surgery. DIRT has been established as a useful tool in diagnosing and monitoring the healing of cold injuries. The group is aslo evaluating DIRT as a clinical tool in pain syndromes caused by nerve entrapment.
Lenke til nyhet
29.06.2023:
Neurogenic pain and vasospasm
The Departments of Radiology and Department of Plastic and Reconstructive Surgery have been cooperating in the treatment of neurogenic pain since 2008.
Neurogenic pain and vasospasm
The Departments of Radiology and Department of Plastic and Reconstructive Surgery have been cooperating in the treatment of neurogenic pain since 2008.
A new technique has been developed for ultrasound-guided injection of botulinum toxin for patients with anterior cutaneous nerve entrapment syndrome (ACNES). Traditionally this syndrome has been treated with surgery, which is effective in approximately 50% of the patients. Botulinum toxin A (BTA) is effective in the treatment of neurogenic pain. However, it is a challenge to localize the affected nerve for precise injection of the drug in proximity of the nerve compression. Based on the research on thermography and perforator flaps, the MIRS has developed a new ultrasound-guided technique using color Doppler to visualize the vessels accompanying the nerves through the muscle and subcutaneous tissue towards the skin. The technique allows for precise positioning of the syringe needle for effective treatment of ACNES. The technique has also been used on several other pain syndromes with promising results.

The research group has developed a new technique for the treatment of Raynaud’s phenomenon and disease involving sympathetic block with BTA around the radial artery. It is known from the literature that 8-9 injections of BTA in the hand is effective to treat vasospasm. However, this technique is painful for the patients. With this new technique only one injection is performed at each wrist, which involves very little pain.
Several research projects are planned on ultrasound-guided intervention, some of these are included in a PhD project.
Lenke til nyhet
29.06.2023:
Dermatoplastic Imaging Research Group
Since 2002 there has been close research collaboration between the Department of Radiology and the Department of Plastic Surgery at the University Hospital of North Norway (UNN). Together with the Department of Dermatology a new research group is founded.
Dermatoplastic Imaging Research Group
Since 2002 there has been close research collaboration between the Department of Radiology and the Department of Plastic Surgery at the University Hospital of North Norway (UNN). Together with the Department of Dermatology a new research group is founded.
The collaboration has resulted in many scientific publications on new surgical techniques such as tissue transfer using involving perforator flaps after cancer surgery and trauma, fat transplantation in the treatment of recalcitrant fistula, new techniques for abdominal wall reconstructions in cases of advanced hernias and new methods for the surgical treatment of new-borns with myelomeningocele. Dynamic infrared thermography (DIRT) has been developed and established as a reliable imaging modality in the pre-, intra- and postoperative phases of perforator flap surgery. New surgical methods have been tested in collaboration with the departments of gastroenterological surgery and neurosurgery and imaging techniques have a central role in this research. New techniques for ultrasound-guided injections of botulinum toxin in pain medicine and the treatment of vasospasm have been developed and are currently being evaluated.
Dermatoplastic Imaging Research Group (DIRG) was established in 2023 in collaboration with the Department of Dermatology at UNN.
Lenke til nyhet